Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat
- PMID: 17717140
- PMCID: PMC1988882
- DOI: 10.2353/ajpath.2007.070199
Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat
Abstract
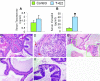
Oxidative and nitrosative stress have been implicated in prostate carcinogenesis, but the cause(s) of redox imbalance in the gland remains poorly defined. We and others have reported that administration of testosterone plus 17beta-estradiol to Noble rats for 16 weeks induces dysplasia and stromal inflammation of the lateral prostate (LP) but not the ventral prostate. Here, using laser capture microdissected specimens, we found that the combined hormone regimen increased the expression of mRNA of specific members of NAD(P)H oxidase (NOX-1, NOX-2, and NOX4), nitric-oxide synthase [NOS; inducible NOS and endothelial NOS], and cyclooxygenase (COX-2) in the LP epithelium and/or its adjacent inflammatory stroma. Accompanying these changes was the accumulation of 8-hydroxy-2'-deoxyguanosine, 4-hydroxynonenal protein adducts, and nitrotyrosine, primarily in the LP epithelium, suggesting that NOX, NOS, and COX may mediate hormone-induced oxidative/nitrosative stress in epithelium. We concluded that the oxidative/nitrosative damage resulting from the testosterone-plus-17beta-estradiol treatment is not solely derived from stromal inflammatory lesions but likely also originates from the epithelium per se. In this context, the up-regulation of COX-2 from epithelium represents a potential mechanism by which the hormone-initiated epithelium might induce inflammatory responses. Thus, we link alterations in the hormonal milieu with oxidative/nitrosative/inflammatory damage to the prostate epithelium that promotes carcinogenesis.
Figures




Similar articles
-
Changes in serum and tissue zinc levels in sex hormone-induced prostatic carcinogenesis in the noble rat.Tumour Biol. 2000 Nov-Dec;21(6):328-36. doi: 10.1159/000030138. Tumour Biol. 2000. PMID: 11006573
-
Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue.Cancer Res. 2000 Nov 1;60(21):6008-17. Cancer Res. 2000. PMID: 11085521
-
Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-I (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer.Prostate. 1998 May 15;35(3):165-77. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. Prostate. 1998. PMID: 9582085
-
Multistage prostate carcinogenesis: the role of hormones.Princess Takamatsu Symp. 1991;22:109-23. Princess Takamatsu Symp. 1991. PMID: 1844235 Review.
-
Role of stroma in carcinogenesis of the prostate.Differentiation. 2002 Dec;70(9-10):473-85. doi: 10.1046/j.1432-0436.2002.700902.x. Differentiation. 2002. PMID: 12492490 Review.
Cited by
-
Nrf2 and NF-κB and Their Concerted Modulation in Cancer Pathogenesis and Progression.Cancers (Basel). 2010 Apr 13;2(2):483-97. doi: 10.3390/cancers2020483. Cancers (Basel). 2010. PMID: 24281078 Free PMC article.
-
The evolutionary impact of androgen levels on prostate cancer in a multi-scale mathematical model.Biol Direct. 2010 Apr 20;5:24. doi: 10.1186/1745-6150-5-24. Biol Direct. 2010. PMID: 20406442 Free PMC article.
-
Polymorphisms of pro-inflammatory genes and prostate cancer risk: a pharmacogenomic approach.Cancer Immunol Immunother. 2009 Dec;58(12):1919-33. doi: 10.1007/s00262-009-0658-y. Epub 2009 Feb 17. Cancer Immunol Immunother. 2009. PMID: 19221747 Free PMC article. Review.
-
A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model.Cancer Res. 2009 Oct 1;69(19):7689-95. doi: 10.1158/0008-5472.CAN-08-2472. Epub 2009 Sep 22. Cancer Res. 2009. PMID: 19773450 Free PMC article.
-
Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells.PLoS One. 2010 Feb 5;5(2):e9075. doi: 10.1371/journal.pone.0009075. PLoS One. 2010. PMID: 20140087 Free PMC article.
References
-
- Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W, Jiang X, Oberley TD. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000;89:123–134. - PubMed
-
- Oberley TD, Zhong W, Szweda LI, Oberley LW. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–155. - PubMed
-
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. - PubMed
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

