Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling
- PMID: 17703191
- PMCID: PMC1994124
- DOI: 10.1038/sj.emboj.7601823
Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling
Abstract
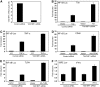
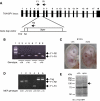
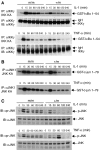
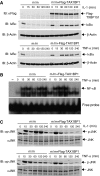
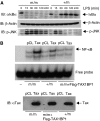
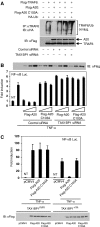
The NF-kappaB transcription factor is normally transiently activated by proinflammatory cytokines and bacterial lipopolysaccharide (LPS); however, persistent NF-kappaB activation is commonly observed in inflammatory disease and malignancy. The ubiquitin editing enzyme A20 serves an essential role in the termination of TNF-alpha- and LPS-mediated NF-kappaB signaling by inactivating key signaling molecules. However, little is known about how A20 is regulated and if other molecules play a role in the termination of NF-kappaB signaling. Here we demonstrate that Tax1-binding protein 1 (TAX1BP1) is essential for the termination of NF-kappaB and JNK activation in response to TNF-alpha, IL-1 and LPS stimulation. In TAX1BP1-deficient mouse fibroblasts, TNF-alpha-, IL-1- and LPS-mediated IKK and JNK activation is elevated and persistent owing to enhanced ubiquitination of RIP1 and TRAF6. Furthermore, in the absence of TAX1BP1, A20 is impaired in RIP1 binding, deubiquitination of TRAF6 and inhibition of NF-kappaB activation. Thus, TAX1BP1 is pivotal for the termination of NF-kappaB and JNK signaling by functioning as an essential regulator of A20.
Figures



Similar articles
-
TRAF6 negatively regulates TNFalpha-induced NF-kappaB activation.Cytokine. 2009 Feb;45(2):72-9. doi: 10.1016/j.cyto.2008.10.010. Epub 2008 Dec 16. Cytokine. 2009. PMID: 19091594
-
The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling.EMBO J. 2009 Mar 4;28(5):513-22. doi: 10.1038/emboj.2008.285. Epub 2009 Jan 8. EMBO J. 2009. PMID: 19131965 Free PMC article.
-
Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis.EMBO J. 2001 Mar 15;20(6):1271-80. doi: 10.1093/emboj/20.6.1271. EMBO J. 2001. PMID: 11250893 Free PMC article.
-
The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology.Trends Immunol. 2009 Aug;30(8):383-91. doi: 10.1016/j.it.2009.05.007. Epub 2009 Jul 28. Trends Immunol. 2009. PMID: 19643665 Review.
-
From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey.Immunol Rev. 2007 Dec;220:8-21. doi: 10.1111/j.1600-065X.2007.00560.x. Immunol Rev. 2007. PMID: 17979836 Review.
Cited by
-
RING finger protein 11 targets TBK1/IKKi kinases to inhibit antiviral signaling.PLoS One. 2013;8(1):e53717. doi: 10.1371/journal.pone.0053717. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308279 Free PMC article.
-
RING finger protein 11 (RNF11) modulates susceptibility to 6-OHDA-induced nigral degeneration and behavioral deficits through NF-κB signaling in dopaminergic cells.Neurobiol Dis. 2013 Jun;54:264-79. doi: 10.1016/j.nbd.2012.12.018. Epub 2013 Jan 11. Neurobiol Dis. 2013. PMID: 23318928 Free PMC article.
-
Multiomics analysis reveals the mechanical stress-dependent changes in trabecular meshwork cytoskeletal-extracellular matrix interactions.Front Cell Dev Biol. 2022 Sep 13;10:874828. doi: 10.3389/fcell.2022.874828. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36176278 Free PMC article.
-
Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system.Immunol Rev. 2015 Jul;266(1):72-92. doi: 10.1111/imr.12302. Immunol Rev. 2015. PMID: 26085208 Free PMC article. Review.
-
USP7-Dependent Regulation of TRAF Activation and Signaling by a Viral Interferon Regulatory Factor Homologue.J Virol. 2020 Jan 6;94(2):e01553-19. doi: 10.1128/JVI.01553-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31666375 Free PMC article.
References
-
- Bertelsen M, Sanfridson A (2007) TAB1 modulates IL-1α mediated cytokine secretion but is dispensable for TAK1 activation. Cell Signal 19: 646–657 - PubMed
-
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 - PubMed
-
- Chin KT, Chun AC, Ching YP, Jeang KT, Jin DY (2007) Human T-cell leukemia virus oncoprotein tax represses nuclear receptor-dependent transcription by targeting coactivator TAX1BP1. Cancer Res 67: 1072–1081 - PubMed
-
- Chun AC, Zhou Y, Wong CM, Kung HF, Jeang KT, Jin DY (2000) Coiled-coil motif as a structural basis for the interaction of HTLV type 1 tax with cellular cofactors. AIDS Res Hum Retroviruses 16: 1689–1694 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

