Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes
- PMID: 17698589
- PMCID: PMC2118695
- DOI: 10.1084/jem.20070204
Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes
Abstract
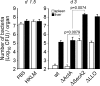
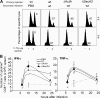
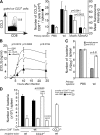
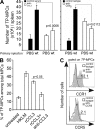
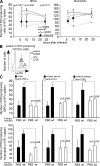
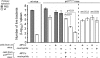
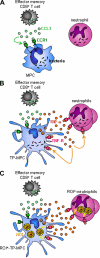
Cytolysis, interferon gamma and tumor necrosis factor (TNF) alpha secretion are major effector mechanisms of memory CD8+ T cells that are believed to be required for immunological protection in vivo. By using mutants of the intracellular bacterium Listeria monocytogenes, we found that none of these effector activities is sufficient to protect against secondary infection with wild-type (WT) bacteria. We demonstrated that CCL3 derived from reactivated memory CD8+ T cells is required for efficient killing of WT bacteria. CCL3 induces a rapid TNF-alpha secretion by innate inflammatory mononuclear phagocytic cells (MPCs), which further promotes the production of radical oxygen intermediates (ROIs) by both MPCs and neutrophils. ROI generation is the final bactericidal mechanism involved in L. monocytogenes clearance. These results therefore uncover two levels of regulation of the antibacterial secondary protective response: (a) an antigen-dependent phase in which memory CD8+ T cells are reactivated and control the activation of the innate immune system, and (b) an antigen-independent phase in which the MPCs coordinate innate immunity and promote the bactericidal effector activities. In this context, CCL3-secreting memory CD8+ T cells are able to mediate "bystander" killing of an unrelated pathogen upon antigen-specific reactivation, a mechanism that may be important for the design of therapeutic vaccines.
Figures



Similar articles
-
Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo.PLoS Pathog. 2011 Dec;7(12):e1002457. doi: 10.1371/journal.ppat.1002457. Epub 2011 Dec 29. PLoS Pathog. 2011. PMID: 22241983 Free PMC article.
-
Activation of antigen-specific CD8 T cells results in minimal killing of bystander bacteria.J Immunol. 2003 Dec 1;171(11):6032-8. doi: 10.4049/jimmunol.171.11.6032. J Immunol. 2003. PMID: 14634115
-
Priming of protective anti-Listeria monocytogenes memory CD8+ T cells requires a functional SecA2 secretion system.Infect Immun. 2011 Jun;79(6):2396-403. doi: 10.1128/IAI.00020-11. Epub 2011 Mar 14. Infect Immun. 2011. PMID: 21402759 Free PMC article.
-
Probing CD8 T cell responses with Listeria monocytogenes infection.Adv Immunol. 2012;113:51-80. doi: 10.1016/B978-0-12-394590-7.00005-1. Adv Immunol. 2012. PMID: 22244579 Review.
-
Listeria monocytogenes: a model pathogen to study antigen-specific memory CD8 T cell responses.Semin Immunopathol. 2015 May;37(3):301-10. doi: 10.1007/s00281-015-0477-5. Epub 2015 Apr 10. Semin Immunopathol. 2015. PMID: 25860798 Free PMC article. Review.
Cited by
-
Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection.PLoS Pathog. 2014 Feb 27;10(2):e1003970. doi: 10.1371/journal.ppat.1003970. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24586170 Free PMC article.
-
T-cell immunity to SARS-CoV-2: what if the known best is not the optimal course for the long run? Adapting to evolving targets.Front Immunol. 2023 Jun 14;14:1133225. doi: 10.3389/fimmu.2023.1133225. eCollection 2023. Front Immunol. 2023. PMID: 37388738 Free PMC article. Review.
-
Trends in cancer immunotherapy.Clin Med Insights Oncol. 2010 Jul 14;4:67-80. doi: 10.4137/cmo.s4795. Clin Med Insights Oncol. 2010. PMID: 20703326 Free PMC article.
-
Monocyte-mediated defense against bacteria, fungi, and parasites.Semin Immunol. 2015 Dec;27(6):397-409. doi: 10.1016/j.smim.2016.03.014. Epub 2016 Mar 25. Semin Immunol. 2015. PMID: 27021645 Free PMC article. Review.
-
Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo.PLoS Pathog. 2011 Dec;7(12):e1002457. doi: 10.1371/journal.ppat.1002457. Epub 2011 Dec 29. PLoS Pathog. 2011. PMID: 22241983 Free PMC article.
References
-
- Harty, J.T., A.R. Tvinnereim, and D.W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308. - PubMed
-
- Lauvau, G., S. Vijh, P. Kong, T. Horng, K. Kerksiek, N. Serbina, R.A. Tuma, and E.G.P. Am. 2001. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 294:1735–1739. - PubMed
-
- Badovinac, V.P., and J.T. Harty. 2000. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma. J. Immunol. 164:6444–6452. - PubMed
-
- Badovinac, V.P., A.R. Tvinnereim, and J.T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 290:1354–1358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

