Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome
- PMID: 17698009
- PMCID: PMC1994817
- DOI: 10.1016/j.neuron.2007.07.020
Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome
Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a recently recognized neurodegenerative disorder in fragile X premutation carriers with FMR1 alleles containing 55-200 CGG repeats. Previously, we developed a Drosophila model of FXTAS and demonstrated that transcribed premutation repeats alone are sufficient to cause neurodegeneration, suggesting that rCGG-repeat-binding proteins (RBPs) may be sequestered from their normal function by rCGG binding. Here, we identify Pur alpha and hnRNP A2/B1 as RBPs. We show that Pur alpha and rCGG repeats interact in a sequence-specific fashion that is conserved between mammals and Drosophila. Overexpression of Pur alpha in Drosophila could suppress rCGG-mediated neurodegeneration in a dose-dependent manner. Furthermore, Pur alpha is also present in the inclusions of FXTAS patient brains. These findings support the disease mechanism of FXTAS of rCGG repeat sequestration of specific RBPs, leading to neuronal cell death, and implicate that Pur alpha plays an important role in the pathogenesis of FXTAS.
Figures
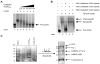
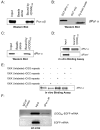
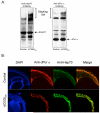


Comment in
-
Fragile X tremor/ataxia syndrome: blame the messenger!Neuron. 2007 Aug 16;55(4):535-7. doi: 10.1016/j.neuron.2007.07.032. Neuron. 2007. PMID: 17698005
Similar articles
-
RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS.Neuron. 2007 Aug 16;55(4):565-71. doi: 10.1016/j.neuron.2007.07.021. Neuron. 2007. PMID: 17698010 Free PMC article.
-
Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by Fragile X premutation rCGG repeats.PLoS Genet. 2011 Jun;7(6):e1002102. doi: 10.1371/journal.pgen.1002102. Epub 2011 Jun 2. PLoS Genet. 2011. PMID: 21655086 Free PMC article.
-
MicroRNA-277 modulates the neurodegeneration caused by Fragile X premutation rCGG repeats.PLoS Genet. 2012;8(5):e1002681. doi: 10.1371/journal.pgen.1002681. Epub 2012 May 3. PLoS Genet. 2012. PMID: 22570635 Free PMC article.
-
RNA-mediated neurodegeneration in fragile X-associated tremor/ataxia syndrome.Brain Res. 2012 Jun 26;1462:112-7. doi: 10.1016/j.brainres.2012.02.057. Epub 2012 Mar 9. Brain Res. 2012. PMID: 22459047 Free PMC article. Review.
-
Fragile X-associated tremor/ataxia syndrome (FXTAS).Ment Retard Dev Disabil Res Rev. 2004;10(1):25-30. doi: 10.1002/mrdd.20005. Ment Retard Dev Disabil Res Rev. 2004. PMID: 14994285 Review.
Cited by
-
Poly(ADP-Ribosyl)ation of hnRNP A1 Protein Controls Translational Repression in Drosophila.Mol Cell Biol. 2016 Sep 12;36(19):2476-86. doi: 10.1128/MCB.00207-16. Print 2016 Oct 1. Mol Cell Biol. 2016. PMID: 27402862 Free PMC article.
-
Repeat expansion disease: progress and puzzles in disease pathogenesis.Nat Rev Genet. 2010 Apr;11(4):247-58. doi: 10.1038/nrg2748. Nat Rev Genet. 2010. PMID: 20177426 Free PMC article. Review.
-
Perturbation of the Akt/Gsk3-β signalling pathway is common to Drosophila expressing expanded untranslated CAG, CUG and AUUCU repeat RNAs.Hum Mol Genet. 2011 Jul 15;20(14):2783-94. doi: 10.1093/hmg/ddr177. Epub 2011 Apr 25. Hum Mol Genet. 2011. PMID: 21518731 Free PMC article.
-
Regulation of molecular pathways in the Fragile X Syndrome: insights into Autism Spectrum Disorders.J Neurodev Disord. 2011 Sep;3(3):257-69. doi: 10.1007/s11689-011-9087-2. Epub 2011 Aug 13. J Neurodev Disord. 2011. PMID: 21842222 Free PMC article.
-
Dysregulation of anti-Mullerian hormone expression levels in mural granulosa cells of FMR1 premutation carriers.Sci Rep. 2021 Jul 8;11(1):14139. doi: 10.1038/s41598-021-93489-x. Sci Rep. 2021. PMID: 34238973 Free PMC article.
References
-
- Arocena DG, Iwahashi CK, Won N, Beilina A, Ludwig AL, Tassone F, Schwartz PH, Hagerman PJ. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14:3661–3671. - PubMed
-
- Deissler H, Behn-Krappa A, Doerfler W. Purification of nuclear proteins from human HeLa cells that bind specifically to the unstable tandem repeat (CGG)n in the human FMR1 gene. J Biol Chem. 1996;271:4327–4334. - PubMed
-
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. - PubMed
-
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, Leehey M, Hagerman PJ. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

