Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle
- PMID: 17696647
- PMCID: PMC1945040
- DOI: 10.1371/journal.pbio.0050220
Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle
Abstract
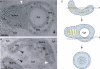
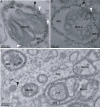
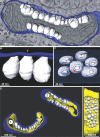
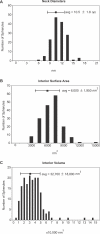
Positive-strand RNA viruses are the largest genetic class of viruses and include many serious human pathogens. All positive-strand RNA viruses replicate their genomes in association with intracellular membrane rearrangements such as single- or double-membrane vesicles. However, the exact sites of RNA synthesis and crucial topological relationships between relevant membranes, vesicle interiors, surrounding lumens, and cytoplasm generally are poorly defined. We applied electron microscope tomography and complementary approaches to flock house virus (FHV)-infected Drosophila cells to provide the first 3-D analysis of such replication complexes. The sole FHV RNA replication factor, protein A, and FHV-specific 5-bromouridine 5'-triphosphate incorporation localized between inner and outer mitochondrial membranes inside approximately 50-nm vesicles (spherules), which thus are FHV-induced compartments for viral RNA synthesis. All such FHV spherules were outer mitochondrial membrane invaginations with interiors connected to the cytoplasm by a necked channel of approximately 10-nm diameter, which is sufficient for ribonucleotide import and product RNA export. Tomographic, biochemical, and other results imply that FHV spherules contain, on average, three RNA replication intermediates and an interior shell of approximately 100 membrane-spanning, self-interacting protein As. The results identify spherules as the site of protein A and nascent RNA accumulation and define spherule topology, dimensions, and stoichiometry to reveal the nature and many details of the organization and function of the FHV RNA replication complex. The resulting insights appear relevant to many other positive-strand RNA viruses and support recently proposed structural and likely evolutionary parallels with retrovirus and double-stranded RNA virus virions.
Conflict of interest statement
Figures


Similar articles
-
Role of Mitochondrial Membrane Spherules in Flock House Virus Replication.J Virol. 2016 Jan 20;90(7):3676-83. doi: 10.1128/JVI.03080-15. J Virol. 2016. PMID: 26792749 Free PMC article.
-
Cryo-electron tomography reveals novel features of a viral RNA replication compartment.Elife. 2017 Jun 27;6:e25940. doi: 10.7554/eLife.25940. Elife. 2017. PMID: 28653620 Free PMC article.
-
Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells.J Virol. 2001 Dec;75(23):11664-76. doi: 10.1128/JVI.75.23.11664-11676.2001. J Virol. 2001. PMID: 11689648 Free PMC article.
-
Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories.Annu Rev Microbiol. 2010;64:241-56. doi: 10.1146/annurev.micro.112408.134012. Annu Rev Microbiol. 2010. PMID: 20825348 Review.
-
Multiscale Electron Microscopy for the Study of Viral Replication Organelles.Viruses. 2021 Jan 28;13(2):197. doi: 10.3390/v13020197. Viruses. 2021. PMID: 33525547 Free PMC article. Review.
Cited by
-
Nodavirus RNA replication crown architecture reveals proto-crown precursor and viral protein A conformational switching.Proc Natl Acad Sci U S A. 2023 Jan 31;120(5):e2217412120. doi: 10.1073/pnas.2217412120. Epub 2023 Jan 24. Proc Natl Acad Sci U S A. 2023. PMID: 36693094 Free PMC article.
-
Multi-color super-resolution imaging to study human coronavirus RNA during cellular infection.Cell Rep Methods. 2022 Feb 28;2(2):100170. doi: 10.1016/j.crmeth.2022.100170. Epub 2022 Feb 1. Cell Rep Methods. 2022. PMID: 35128513 Free PMC article.
-
In vitro and in vivo characterization of microRNA-targeted alphavirus replicon and helper RNAs.J Virol. 2010 Aug;84(15):7713-25. doi: 10.1128/JVI.00310-10. Epub 2010 May 26. J Virol. 2010. PMID: 20504925 Free PMC article.
-
Architecture and biogenesis of plus-strand RNA virus replication factories.World J Virol. 2013 May 12;2(2):32-48. doi: 10.5501/wjv.v2.i2.32. World J Virol. 2013. PMID: 24175228 Free PMC article. Review.
-
Mitochondrion-enriched anionic phospholipids facilitate flock house virus RNA polymerase membrane association.J Virol. 2009 May;83(9):4498-507. doi: 10.1128/JVI.00040-09. Epub 2009 Feb 25. J Virol. 2009. PMID: 19244330 Free PMC article.
References
-
- van Regenmortel MHV, editor. Virus taxonomy. San Diego: Academic Press; 2000. 1162
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

