TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways
- PMID: 17694177
- PMCID: PMC1937497
- DOI: 10.1172/JCI29134
TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways
Abstract
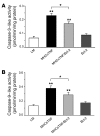
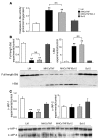
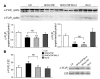
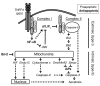
Transgenic mice with cardiac-restricted overexpression of secretable TNF (MHCsTNF) develop progressive LV wall thinning and dilation accompanied by an increase in cardiomyocyte apoptosis and a progressive loss of cytoprotective Bcl-2. To test whether cardiac-restricted overexpression of Bcl-2 would prevent adverse cardiac remodeling, we crossed MHCsTNF mice with transgenic mice harboring cardiac-restricted overexpression of Bcl-2. Sustained TNF signaling resulted in activation of the intrinsic cell death pathway, leading to increased cytosolic levels of cytochrome c, Smac/Diablo and Omi/HtrA2, and activation of caspases -3 and -9. Cardiac-restricted overexpression of Bcl-2 blunted activation of the intrinsic pathway and prevented LV wall thinning; however, Bcl-2 only partially attenuated cardiomyocyte apoptosis. Subsequent studies showed that c-FLIP was degraded, that caspase-8 was activated, and that Bid was cleaved to t-Bid, suggesting that the extrinsic pathway was activated concurrently in MHCsTNF hearts. As expected, cardiac Bcl-2 overexpression had no effect on extrinsic signaling. Thus, our results suggest that sustained inflammation leads to activation of multiple cell death pathways that contribute to progressive cardiomyocyte apoptosis; hence the extent of such programmed myocyte cell death is a critical determinant of adverse cardiac remodeling.
Figures

Similar articles
-
Cardiac myocyte apoptosis provokes adverse cardiac remodeling in transgenic mice with targeted TNF overexpression.Am J Physiol Heart Circ Physiol. 2004 Sep;287(3):H1303-11. doi: 10.1152/ajpheart.00053.2004. Am J Physiol Heart Circ Physiol. 2004. PMID: 15317679
-
Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells.Oncogene. 2004 Jun 3;23(26):4523-35. doi: 10.1038/sj.onc.1207594. Oncogene. 2004. PMID: 15064710
-
Caspase-9 is activated in a cytochrome c-independent manner early during TNFalpha-induced apoptosis in murine cells.Cell Death Differ. 2003 Sep;10(9):1005-15. doi: 10.1038/sj.cdd.4401271. Cell Death Differ. 2003. PMID: 12934075
-
Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways.Cell Res. 2000 Sep;10(3):161-7. doi: 10.1038/sj.cr.7290045. Cell Res. 2000. PMID: 11032168 Review.
-
Regulation of cardiac myocyte cell death.Pharmacol Ther. 2003 Mar;97(3):223-61. doi: 10.1016/s0163-7258(02)00339-x. Pharmacol Ther. 2003. PMID: 12576135 Review.
Cited by
-
Electroimmunology and cardiac arrhythmia.Nat Rev Cardiol. 2021 Aug;18(8):547-564. doi: 10.1038/s41569-021-00520-9. Epub 2021 Mar 2. Nat Rev Cardiol. 2021. PMID: 33654273 Free PMC article. Review.
-
Tumor necrosis factor receptor-associated factor 2 signaling provokes adverse cardiac remodeling in the adult mammalian heart.Circ Heart Fail. 2013 May;6(3):535-43. doi: 10.1161/CIRCHEARTFAILURE.112.000080. Epub 2013 Mar 14. Circ Heart Fail. 2013. PMID: 23493088 Free PMC article.
-
Cardiovascular manifestations of inflammatory bowel diseases and the underlying pathogenic mechanisms.Am J Physiol Regul Integr Comp Physiol. 2023 Aug 1;325(2):R193-R211. doi: 10.1152/ajpregu.00300.2022. Epub 2023 Jun 19. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 37335014 Free PMC article. Review.
-
Microparticles from apoptotic RAW 264.7 macrophage cells carry tumour necrosis factor-α functionally active on cardiomyocytes from adult mice.J Extracell Vesicles. 2015 Oct 23;4:28621. doi: 10.3402/jev.v4.28621. eCollection 2015. J Extracell Vesicles. 2015. PMID: 26498917 Free PMC article.
-
Evaluation of Oxidative Stress, Apoptosis, and Expression of MicroRNA-208a and MicroRNA-1 in Cardiovascular Patients.Rep Biochem Mol Biol. 2021 Jul;10(2):183-196. doi: 10.52547/rbmb.10.2.183. Rep Biochem Mol Biol. 2021. PMID: 34604408 Free PMC article.
References
-
- Sivasubramanian N., et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. - PubMed
-
- Bryant D., et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α (TNF). Circulation. 1998;97:1375–1381. - PubMed
-
- Kubota T., et al. Dilated cardiomyopathy in transgenic mice with cardiac specific overexpression of tumor necrosis factor-alpha. Circ. Res. 1997;81:627–635. - PubMed
-
- Engel D., Peshock R., Armstrong R.C., Sivasubramanian N., Mann D.L. Cardiac myocyte apoptosis provokes adverse cardiac remodeling in transgenic mice with targeted TNF overexpression. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1303–H1311. - PubMed
-
- Bishopric N.H., Andreka P., Slepak T., Webster K.A. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr. Opin. Pharmacol. 2001;1:141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

