Bacterial sensor kinase TodS interacts with agonistic and antagonistic signals
- PMID: 17693554
- PMCID: PMC1959458
- DOI: 10.1073/pnas.0701547104
Bacterial sensor kinase TodS interacts with agonistic and antagonistic signals
Abstract
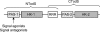
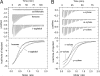
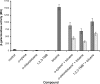
The TodS/TodT two-component system controls expression of the toluene dioxygenase (TOD) pathway for the metabolism of toluene in Pseudomonas putida DOT-T1E. TodS is a sensor kinase that ultimately controls tod gene expression through its cognate response regulator, TodT. We used isothermal titration calorimetry to study the binding of different compounds to TodS and related these findings to their capacity to induce gene expression in vivo. Agonistic compounds bound to TodS and induced gene expression in vivo. Toluene was a powerful agonist, but ortho-substitutions of toluene reduced or abolished in vivo responses, although TodS recognized o-xylene with high affinity. These compounds were called antagonists. We show that agonists and antagonists compete for binding to TodS both in vitro and in vivo. The failure of antagonists to induce gene expression in vivo correlated with their inability to stimulate TodS autophosphorylation in vitro. We propose intramolecular TodS signal transmission, not molecular recognition of compounds by TodS, to be the phenomenon that determines whether a given compound will lead to activation of expression of the tod genes. Molecular modeling identified residues F46, I74, F79, and I114 as being potentially involved in the binding of effector molecules. Alanine substitution mutants of these residues reduced affinities (2- to 345-fold) for both agonistic and antagonistic compounds. Our data indicate that determining the inhibitory activity of antagonists is a potentially fruitful alternative to design specific two-component system inhibitors for the development of new drugs to inhibit processes regulated by two-component systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Construction of a prototype two-component system from the phosphorelay system TodS/TodT.Protein Eng Des Sel. 2012 Apr;25(4):159-69. doi: 10.1093/protein/gzs001. Epub 2012 Feb 3. Protein Eng Des Sel. 2012. PMID: 22308529
-
Hierarchical binding of the TodT response regulator to its multiple recognition sites at the tod pathway operon promoter.J Mol Biol. 2008 Feb 15;376(2):325-37. doi: 10.1016/j.jmb.2007.12.004. Epub 2007 Dec 8. J Mol Biol. 2008. PMID: 18166197
-
The TodS-TodT two-component regulatory system recognizes a wide range of effectors and works with DNA-bending proteins.Proc Natl Acad Sci U S A. 2006 May 23;103(21):8191-6. doi: 10.1073/pnas.0602902103. Epub 2006 May 15. Proc Natl Acad Sci U S A. 2006. PMID: 16702539 Free PMC article.
-
Molecular Insights into Toluene Sensing in the TodS/TodT Signal Transduction System.J Biol Chem. 2016 Apr 15;291(16):8575-90. doi: 10.1074/jbc.M116.718841. Epub 2016 Feb 22. J Biol Chem. 2016. PMID: 26903514 Free PMC article.
-
Multiple signals modulate the activity of the complex sensor kinase TodS.Microb Biotechnol. 2015 Jan;8(1):103-15. doi: 10.1111/1751-7915.12142. Epub 2014 Jul 1. Microb Biotechnol. 2015. PMID: 24986263 Free PMC article.
Cited by
-
Advances in ligand-specific biosensing for structurally similar molecules.Cell Syst. 2023 Dec 20;14(12):1024-1043. doi: 10.1016/j.cels.2023.10.009. Cell Syst. 2023. PMID: 38128482 Review.
-
Genetic Analysis Reveals a Requirement for the Hybrid Sensor Kinase RscS in para-Aminobenzoic Acid/Calcium-Induced Biofilm Formation by Vibrio fischeri.J Bacteriol. 2023 Jul 25;205(7):e0007523. doi: 10.1128/jb.00075-23. Epub 2023 Jun 12. J Bacteriol. 2023. PMID: 37306594 Free PMC article.
-
Emergence of an Auxin Sensing Domain in Plant-Associated Bacteria.mBio. 2023 Feb 28;14(1):e0336322. doi: 10.1128/mbio.03363-22. Epub 2023 Jan 5. mBio. 2023. PMID: 36602305 Free PMC article.
-
Flagella, Chemotaxis and Surface Sensing.Adv Exp Med Biol. 2022;1386:185-221. doi: 10.1007/978-3-031-08491-1_7. Adv Exp Med Biol. 2022. PMID: 36258073
-
Fluoro-recognition: New in vivo fluorescent assay for toluene dioxygenase probing induction by and metabolism of polyfluorinated compounds.Environ Microbiol. 2022 Nov;24(11):5202-5216. doi: 10.1111/1462-2920.16187. Epub 2022 Oct 17. Environ Microbiol. 2022. PMID: 36054238 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

