Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin
- PMID: 17685442
- PMCID: PMC4364136
- DOI: 10.1002/cm.20226
Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin
Abstract
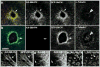
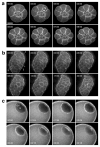
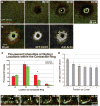
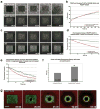
Actin filaments (F-actin) are protein polymers that undergo rapid assembly and disassembly and control an enormous variety of cellular processes ranging from force production to regulation of signal transduction. Consequently, imaging of F-actin has become an increasingly important goal for biologists seeking to understand how cells and tissues function. However, most of the available means for imaging F-actin in living cells suffer from one or more biological or experimental shortcomings. Here we describe fluorescent F-actin probes based on the calponin homology domain of utrophin (Utr-CH), which binds F-actin without stabilizing it in vitro. We show that these probes faithfully report the distribution of F-actin in living and fixed cells, distinguish between stable and dynamic F-actin, and have no obvious effects on processes that depend critically on the balance of actin assembly and disassembly.
(c) 2007 Wiley-Liss, Inc.
Figures

Similar articles
-
The actin binding affinity of the utrophin tandem calponin-homology domain is primarily determined by its N-terminal domain.Biochemistry. 2014 Mar 25;53(11):1801-9. doi: 10.1021/bi500149q. Epub 2014 Mar 14. Biochemistry. 2014. PMID: 24628267
-
The N-Terminal Flanking Region Modulates the Actin Binding Affinity of the Utrophin Tandem Calponin-Homology Domain.Biochemistry. 2017 May 23;56(20):2627-2636. doi: 10.1021/acs.biochem.6b01117. Epub 2017 May 10. Biochemistry. 2017. PMID: 28443334
-
The N- and C-Terminal Domains Differentially Contribute to the Structure and Function of Dystrophin and Utrophin Tandem Calponin-Homology Domains.Biochemistry. 2015 Nov 24;54(46):6942-50. doi: 10.1021/acs.biochem.5b00969. Epub 2015 Nov 13. Biochemistry. 2015. PMID: 26516677
-
Actin-depolymerizing factor homology domain: a conserved fold performing diverse roles in cytoskeletal dynamics.Cytoskeleton (Hoboken). 2011 Sep;68(9):471-90. doi: 10.1002/cm.20530. Epub 2011 Sep 13. Cytoskeleton (Hoboken). 2011. PMID: 21850706 Review.
-
An open or closed case for the conformation of calponin homology domains on F-actin?J Muscle Res Cell Motil. 2004;25(4-5):351-8. doi: 10.1007/s10974-004-0690-7. J Muscle Res Cell Motil. 2004. PMID: 15548864 Review.
Cited by
-
In vivo imaging and characterization of actin microridges.PLoS One. 2015 Jan 28;10(1):e0115639. doi: 10.1371/journal.pone.0115639. eCollection 2015. PLoS One. 2015. PMID: 25629723 Free PMC article.
-
Permeabilization activated reduction in fluorescence: A novel method to measure kinetics of protein interactions with intracellular structures.Cytoskeleton (Hoboken). 2016 Jun;73(6):271-85. doi: 10.1002/cm.21306. Epub 2016 May 24. Cytoskeleton (Hoboken). 2016. PMID: 27126922 Free PMC article.
-
The diffusion of normal skin wound myofibroblast-derived microvesicles differs according to matrix composition.J Extracell Biol. 2023 Dec 27;3(1):e131. doi: 10.1002/jex2.131. eCollection 2024 Jan. J Extracell Biol. 2023. PMID: 38938680 Free PMC article.
-
Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish.Dev Cell. 2010 Feb 16;18(2):226-36. doi: 10.1016/j.devcel.2009.11.015. Dev Cell. 2010. PMID: 20159593 Free PMC article.
-
Stabilization of the actomyosin ring enables spermatocyte cytokinesis in Drosophila.Mol Biol Cell. 2010 May 1;21(9):1482-93. doi: 10.1091/mbc.e09-08-0714. Epub 2010 Mar 17. Mol Biol Cell. 2010. PMID: 20237160 Free PMC article.
References
-
- Aizawa H, Sameshima M, Yahara I. A green fluorescent protein–actin fusion protein dominantly inhibits cytokinesis, cell spreading, and locomotion in Dictyostelium. Cell Struct Funct. 1997;22:335–345. - PubMed
-
- Bement WM, Sokac AM, Mandato CA. Four-dimensional imaging of cytoskeletal dynamics in Xenopus oocytes and eggs. Differentiation. 2003;71:518–527. - PubMed
-
- Dancker P, Low I, Hasselbach W, Wieland T. Interaction of actin with phalloidin: Polymerization and stabilization of F-actin. Biochim Biophys Acta. 1975;400:407–414. - PubMed
-
- Danilchik MV, Funk WC, Brown EE, Larkin K. Requirement for microtubules in new membrane formation during cytokinesis of Xenopus embryos. Dev Biol. 1998;194:47–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

