Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling
- PMID: 17675102
- PMCID: PMC2697967
- DOI: 10.1016/j.neuropharm.2007.06.004
Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling
Abstract
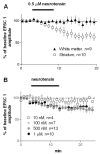
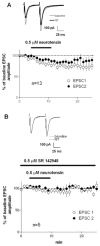
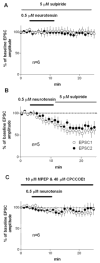
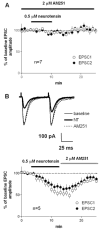
Neurotensin is a peptide that has been suggested to mimic the actions of antipsychotics, but little is known about how it affects synaptic transmission in the striatum, the major input nucleus of the basal ganglia. In this study we measured the effects of neurotensin on EPSCs from medium spiny projection neurons in the sensorimotor striatum, a region implicated in habit formation and control of motor sequences. We found that bath-applied neurotensin reduced glutamate release from presynaptic terminals, and that this effect required retrograde endocannabinoid signaling, as it was prevented by the CB1 cannabinoid receptor antagonist AM251. Neurotensin-mediated inhibition of striatal EPSCs was also blocked by antagonists of D2-like dopamine receptors and group I metabotropic glutamate receptors, as well as by intracellular calcium chelation and phospholipase C inhibition. These results suggest that neurotensin can indirectly engage an endocannabinoid-mediated negative feedback signal to control glutamatergic input to the basal ganglia.
Figures
Similar articles
-
Neurotensin inhibition of GABAergic transmission via mGluR-induced endocannabinoid signalling in rat periaqueductal grey.J Physiol. 2009 Jun 1;587(Pt 11):2511-20. doi: 10.1113/jphysiol.2008.167429. Epub 2009 Apr 9. J Physiol. 2009. PMID: 19359367 Free PMC article.
-
Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum.J Neurosci. 2005 Nov 9;25(45):10537-45. doi: 10.1523/JNEUROSCI.2959-05.2005. J Neurosci. 2005. PMID: 16280591 Free PMC article.
-
Neurotensin selectively facilitates glutamatergic transmission in globus pallidus.Neuroscience. 2006 Sep 15;141(4):1871-8. doi: 10.1016/j.neuroscience.2006.05.049. Epub 2006 Jul 11. Neuroscience. 2006. PMID: 16814931
-
Modulation of endocannabinoid-mediated long-lasting disinhibition of striatal output by cholinergic interneurons.Neuropharmacology. 2011 Dec;61(8):1314-20. doi: 10.1016/j.neuropharm.2011.07.039. Epub 2011 Aug 5. Neuropharmacology. 2011. PMID: 21839753
-
Diverse actions of the modulatory peptide neurotensin on central synaptic transmission.Eur J Neurosci. 2019 Mar;49(6):784-793. doi: 10.1111/ejn.13858. Epub 2018 Feb 28. Eur J Neurosci. 2019. PMID: 29405480 Free PMC article. Review.
Cited by
-
Neurotensin Induces Presynaptic Depression of D2 Dopamine Autoreceptor-Mediated Neurotransmission in Midbrain Dopaminergic Neurons.J Neurosci. 2015 Aug 5;35(31):11144-52. doi: 10.1523/JNEUROSCI.3816-14.2015. J Neurosci. 2015. PMID: 26245975 Free PMC article.
-
Neurotensin agonist attenuates nicotine potentiation to cocaine sensitization.Behav Sci (Basel). 2014 Jan 22;4(1):42-52. doi: 10.3390/bs4010042. eCollection 2014 Mar. Behav Sci (Basel). 2014. PMID: 25379267 Free PMC article.
-
Neurotensin speeds inhibition of dopamine neurons through temporal modulation of GABAA and GABAB receptor-mediated synaptic input.Neuropharmacology. 2018 Mar 15;131:414-423. doi: 10.1016/j.neuropharm.2018.01.004. Epub 2018 Jan 5. Neuropharmacology. 2018. PMID: 29307543 Free PMC article.
-
Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: beneficial actions in an Alzheimer's disease model.J Neurosci. 2014 May 14;34(20):7027-42. doi: 10.1523/JNEUROSCI.0408-14.2014. J Neurosci. 2014. PMID: 24828655 Free PMC article.
-
Neurotensin inhibition of GABAergic transmission via mGluR-induced endocannabinoid signalling in rat periaqueductal grey.J Physiol. 2009 Jun 1;587(Pt 11):2511-20. doi: 10.1113/jphysiol.2008.167429. Epub 2009 Apr 9. J Physiol. 2009. PMID: 19359367 Free PMC article.
References
-
- Battaini F, Govoni S, Di Giovine S, Trabucchi M. Neurotensin effect on dopamine release and calcium transport in rat striatum: interactions with diphenylalkylamine calcium antagonists. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:267–270. - PubMed
-
- Belmeguenai A, Desrues L, Leprince J, Vaudry H, Tonon MC, Louiset E. Neurotensin stimulates both calcium mobilization from inositol trisphosphate-sensitive intracellular stores and calcium influx through membrane channels in frog pituitary melanotrophs. Endocrinology. 2003;144:5556–5567. - PubMed
-
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. - PubMed
-
- Caceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

