Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages
- PMID: 17673941
- PMCID: PMC1936982
- DOI: 10.2119/2007-00019.Miksa
Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages
Abstract
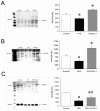
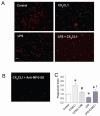
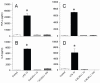
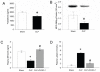
Clearance of apoptotic cells is crucial to maintain cellular function under normal and pathological conditions. We have recently shown that administration of immature dendritic cell-derived exosomes to septic animals promotes phagocytosis of apoptotic cells and improves survival by providing milk fat globule epidermal growth factor-factor VIII (MFG-E8). MFG-E8 acts as an opsonin for apoptotic cells to be engulfed by phagocytosis. In the present study we investigated whether the CX(3)C-chemokine fractalkine (CX(3)CL1) promotes apoptotic cell clearance through the induction of MFG-E8 in peritoneal macrophages. Cultured rat peritoneal macrophages (pMphi) and RAW264.7 macrophages were stimulated with LPS and CX(3)CL1. MFG-E8 expression was assessed by Western blot, cytokine secretion was assessed by ELISA, and phagocytosis of apoptotic thymocytes was determined by microscopy. For in vivo studies, cecal ligation and puncture (CLP) was used to induce sepsis in rats and mice. LPS significantly decreased MFG-E8 levels and phagocytosis of apoptotic cells, whereas CX(3)CL1 induced MFG-E8 expression in both nonstimulated and LPS-stimulated pMphi, without affecting TNF-alpha and IL-6 release. Anti-MFG-E8 blocking antibodies completely abrogated the prophagocytic effect of CX(3)CL1. Twenty hours after the induction of sepsis in rats via CLP, plasma CX(3)CL1 levels as well as MFG-E8 production in peritoneal macrophages decreased by 21% and 56%, respectively. Administration of CX(3)CL1 on the other hand induced MFG-E8 and prevented tissue injury. We conclude that CX(3)CL1 induces MFG-E8 in vitro and in vivo and enhances clearance of apoptotic cells in an MFG-E8-dependent manner. These findings suggest a possible novel treatment for patients in sepsis.
Figures

Similar articles
-
Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis.Shock. 2006 Jun;25(6):586-93. doi: 10.1097/01.shk.0000209533.22941.d0. Shock. 2006. PMID: 16721266
-
Expression and characterization of recombinant human milk fat globule-EGF factor VIII.Int J Mol Med. 2011 Dec;28(6):1071-6. doi: 10.3892/ijmm.2011.782. Epub 2011 Aug 26. Int J Mol Med. 2011. PMID: 21874225
-
Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway.J Immunol. 2009 Jan 1;182(1):581-7. doi: 10.4049/jimmunol.182.1.581. J Immunol. 2009. PMID: 19109191 Free PMC article.
-
MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure.Curr Dir Autoimmun. 2006;9:162-72. doi: 10.1159/000090780. Curr Dir Autoimmun. 2006. PMID: 16394660 Review.
-
Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation.Apoptosis. 2011 Nov;16(11):1077-86. doi: 10.1007/s10495-011-0630-0. Apoptosis. 2011. PMID: 21901532 Review.
Cited by
-
The Apoptosis Paradox in Cancer.Int J Mol Sci. 2022 Jan 25;23(3):1328. doi: 10.3390/ijms23031328. Int J Mol Sci. 2022. PMID: 35163253 Free PMC article. Review.
-
Hijacking homeostasis: Regulation of the tumor microenvironment by apoptosis.Immunol Rev. 2023 Oct;319(1):100-127. doi: 10.1111/imr.13259. Epub 2023 Aug 8. Immunol Rev. 2023. PMID: 37553811 Free PMC article. Review.
-
Sinister self-sacrifice: the contribution of apoptosis to malignancy.Front Immunol. 2014 Jul 4;5:299. doi: 10.3389/fimmu.2014.00299. eCollection 2014. Front Immunol. 2014. PMID: 25071762 Free PMC article. Review. No abstract available.
-
The battle within: cell death by phagocytosis in cancer.Clin Transl Oncol. 2024 Aug 21. doi: 10.1007/s12094-024-03650-x. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39167272 Review.
-
Chemokines and phosphatidylserine: New binding partners for apoptotic cell clearance.Front Cell Dev Biol. 2022 Aug 25;10:943590. doi: 10.3389/fcell.2022.943590. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092729 Free PMC article. No abstract available.
References
-
- Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–7. - PubMed
-
- Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. - PubMed
-
- Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. - PubMed
-
- Shiraishi K, Fukuda S, Mori T, et al. Identification of fractalkine, a CX3C-type chemokine, as a direct target of p53. Cancer Res. 2000;60:3722–6. - PubMed
-
- Garton KJ, Gough PJ, Blobel CP, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–8001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
