Activation of human monocytes after infection by human coronavirus 229E
- PMID: 17669539
- PMCID: PMC7114174
- DOI: 10.1016/j.virusres.2007.06.016
Activation of human monocytes after infection by human coronavirus 229E
Abstract
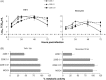
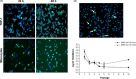
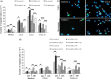
Human coronaviruses (HCoV) are recognized respiratory pathogens that may be involved in other pathologies such as central nervous system (CNS) diseases. To investigate whether leukocytes could participate in respiratory pathologies and serve as vector for viral spread towards other tissues, the susceptibility of human leukocytic cell lines and peripheral blood mononuclear cells (PBMC) to HCoV-229E and HCoV-OC43 infection was investigated. Human primary monocytes/macrophages were susceptible to HCoV-229E infection, but strongly restricted HCoV-OC43 replication. Moreover, productive HCoV-229E infection of primary monocytes and of the THP-1 monocytic cell line led to their activation, as indicated by the production of pro-inflammatory mediators, including TNF-alpha, CCL5, CXCL10 and CXCL11 and MMP-9. Moreover, an in vitro chemotaxis assay showed that motility towards chemokines of THP-1 cells and primary monocytes was increased following an acute or persistent HCoV-229E infection. Taken together, these results suggest that infected monocytes could serve as a reservoir for HCoV-229E, become activated, participate in the exacerbation of pulmonary pathologies, as well as serve as potential vectors for viral dissemination to host tissues, where it could be associated with other pathologies.
Figures



Similar articles
-
A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes.J Virol. 2012 Jul;86(14):7577-87. doi: 10.1128/JVI.00269-12. Epub 2012 May 2. J Virol. 2012. PMID: 22553325 Free PMC article.
-
Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients.J Med Virol. 2006 Jul;78(7):938-49. doi: 10.1002/jmv.20645. J Med Virol. 2006. PMID: 16721849 Free PMC article.
-
Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43.J Infect Dis. 2005 Jun 15;191(12):2033-7. doi: 10.1086/430355. Epub 2005 May 6. J Infect Dis. 2005. PMID: 15897988 Free PMC article.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
-
Antiviral strategies against human coronaviruses.Infect Disord Drug Targets. 2007 Mar;7(1):59-66. doi: 10.2174/187152607780090757. Infect Disord Drug Targets. 2007. PMID: 17346212 Review.
Cited by
-
Role of Monocytes/Macrophages in Covid-19 Pathogenesis: Implications for Therapy.Infect Drug Resist. 2020 Jul 22;13:2485-2493. doi: 10.2147/IDR.S258639. eCollection 2020. Infect Drug Resist. 2020. PMID: 32801787 Free PMC article. Review.
-
A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes.J Virol. 2012 Jul;86(14):7577-87. doi: 10.1128/JVI.00269-12. Epub 2012 May 2. J Virol. 2012. PMID: 22553325 Free PMC article.
-
The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2.Trends Neurosci. 2022 May;45(5):358-368. doi: 10.1016/j.tins.2022.02.006. Epub 2022 Mar 3. Trends Neurosci. 2022. PMID: 35279295 Free PMC article. Review.
-
Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System?Viruses. 2019 Dec 20;12(1):14. doi: 10.3390/v12010014. Viruses. 2019. PMID: 31861926 Free PMC article. Review.
-
Immune Signature of COVID-19: In-Depth Reasons and Consequences of the Cytokine Storm.Int J Mol Sci. 2022 Apr 20;23(9):4545. doi: 10.3390/ijms23094545. Int J Mol Sci. 2022. PMID: 35562935 Free PMC article. Review.
References
-
- Altmeyer R. Virus attachment and entry offer numerous targets for antiviral therapy. Curr. Pharm. Des. 2004;10:3701–3712. - PubMed
-
- Ashmun R.A., Look A.T. Metalloprotease activity of CD13/aminopeptidase N on the surface of myeloid cells. Blood. 1990;75:462–469. - PubMed
-
- Bar-Or A., Nuttall R.K., Duddy M., Alter A., Kim H.J., Ifergan I., Pennington C.J., Bourgoin P., Edwards D.R., Yong V.W. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. - PubMed
-
- Calderon T.M., Eugenin E.A., Lopez L., Kumar S.S., Hesselgesser J., Raine C.S., Berman J.W. A role for CXCL12 (SDF-1α) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J. Neuroimmunol. 2006;177:27–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

