Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells
- PMID: 17668070
- PMCID: PMC1933253
- DOI: 10.1371/journal.pone.0000693
Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells
Abstract
Background: A major obstacle for successful cancer treatment often is the development of drug resistance in cancer cells during chemotherapy. Therefore, there is an urgent need for novel drugs with improved efficacy against tumor cells and with less toxicity on normal cells. Artesunate (ART), a powerful anti-malarial herbal compound, has been shown to inhibit growth of various tumor cell lines in vitro and of xenografted Kaposi's sarcoma in mice in vivo. However, the molecular mechanisms by which ART exerts its cytotoxicity have not been elucidated. The ART-class of anti-malarial compounds is attractive due to their activity against multidrug-resistant Plasmodium falciparum and Plasmodium vivax strains. Another salient feature of these compounds is the lack of severe side effects in malaria patients.
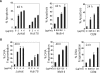
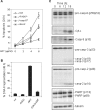
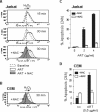
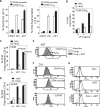
Methodology and principal findings: In this study, we used T-cell leukemias as a model system to study the molecular mechanisms of ART-induced apoptosis. The most typical anticancer drugs are DNA intercalators such as Doxorubicin. To investigate drug sensitivity and resistance, we chose a Doxorubicin-resistant leukemia cell line and investigated the killing effect of ART on these cells. We show that ART induces apoptosis in leukemic T cells mainly through the mitochondrial pathway via generation of reactive oxygen species (ROS), a mechanism different from Doxorubicin. This is confirmed by the fact that the antioxidant N-Acetyle-Cysteine (NAC) could completely block ROS generation and, consequently, inhibited ART-induced apoptosis. Therefore, ART can overcome the Doxorubicin-resistance and induce the Doxorubicin-resistant leukemia cells to undergo apoptosis. We also show that ART can synergize with Doxorubicin to enhance apoptotic cell death in leukemic T cells. This synergistic effect can be largely explained by the fact that ART and Doxorubicin use different killing mechanisms.
Conclusions: Our studies raise the possibility to develop ART in combination with other established anticancer drugs which induce apoptosis through the pathways or mechanisms different from ART.
Conflict of interest statement
Figures
Similar articles
-
Redox active copper chelate overcomes multidrug resistance in T-lymphoblastic leukemia cell by triggering apoptosis.Mol Biosyst. 2011 May;7(5):1701-12. doi: 10.1039/c0mb00306a. Epub 2011 Mar 15. Mol Biosyst. 2011. PMID: 21409205
-
Overcoming multidrug resistance (MDR) in cancer in vitro and in vivo by a quinoline derivative.Biomed Pharmacother. 2011 Sep;65(6):387-94. doi: 10.1016/j.biopha.2011.04.024. Epub 2011 Jun 12. Biomed Pharmacother. 2011. PMID: 21715129
-
The role of reactive oxygen species in WP 631-induced death of human ovarian cancer cells: a comparison with the effect of doxorubicin.Toxicol In Vitro. 2011 Dec;25(8):1712-20. doi: 10.1016/j.tiv.2011.08.009. Epub 2011 Aug 27. Toxicol In Vitro. 2011. PMID: 21896327
-
Artemisinin and its derivatives: a potential treatment for leukemia.Anticancer Drugs. 2019 Jan;30(1):1-18. doi: 10.1097/CAD.0000000000000697. Anticancer Drugs. 2019. PMID: 30540593 Review.
-
Artemisinins: pharmacological actions beyond anti-malarial.Pharmacol Ther. 2014 Apr;142(1):126-39. doi: 10.1016/j.pharmthera.2013.12.001. Epub 2013 Dec 6. Pharmacol Ther. 2014. PMID: 24316259 Review.
Cited by
-
Development of artemisinin compounds for cancer treatment.Invest New Drugs. 2013 Feb;31(1):230-46. doi: 10.1007/s10637-012-9873-z. Epub 2012 Aug 31. Invest New Drugs. 2013. PMID: 22935909 Review.
-
Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer.Med Sci (Basel). 2018 Feb 27;6(1):19. doi: 10.3390/medsci6010019. Med Sci (Basel). 2018. PMID: 29495461 Free PMC article. Review.
-
Artesunate Affects T Antigen Expression and Survival of Virus-Positive Merkel Cell Carcinoma.Cancers (Basel). 2020 Apr 9;12(4):919. doi: 10.3390/cancers12040919. Cancers (Basel). 2020. PMID: 32283634 Free PMC article.
-
Dihydroartemisinin is a Hypoxia-Active Anti-Cancer Drug in Colorectal Carcinoma Cells.Front Oncol. 2014 May 19;4:116. doi: 10.3389/fonc.2014.00116. eCollection 2014. Front Oncol. 2014. PMID: 24904829 Free PMC article.
-
Chalcone-Induced Apoptosis through Caspase-Dependent Intrinsic Pathways in Human Hepatocellular Carcinoma Cells.Int J Mol Sci. 2016 Feb 22;17(2):260. doi: 10.3390/ijms17020260. Int J Mol Sci. 2016. PMID: 26907262 Free PMC article.
References
-
- Rhoads CP. Report on a cooperative study of nitrogen mustard (HN2) trherapy of neoplastic disesase. Trans Assoc Am Physicians. 1947;60:110–111. - PubMed
-
- Boik J. 2001. Natural Compounds in Cancer Therapy, ISBN 0-9648280-1-4, Oregon Medical Press, LLC
-
- Newman DJ, Cragg GM, Snader KM. Natural Products as Sources of New Drugs over the Period 1981-2002. J Nat Prod. 2003;66:1022–1037. - PubMed
-
- Abelson HP. Medicine from Plants. Science. 1990;247:513. - PubMed
-
- Nosten F, Price RN. New antimalarials: a risk-benefit analysis. Drug Saf. 1995;12:264–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

