Prevention of amyloid-like aggregation as a driving force of protein evolution
- PMID: 17668004
- PMCID: PMC1978086
- DOI: 10.1038/sj.embor.7401034
Prevention of amyloid-like aggregation as a driving force of protein evolution
Abstract
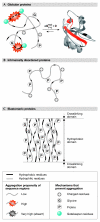
Uncontrolled protein aggregation is a constant challenge in all compartments of living organisms. The failure of a peptide or protein to remain soluble often results in pathology. So far, more than 40 human diseases have been associated with the formation of extracellular fibrillar aggregates - known as amyloid fibrils - or structurally related intracellular deposits. It is well known that molecular chaperones and elaborate quality control mechanisms exist in the cell to counteract aggregation. However, an increasing number of reports during the past few years indicate that proteins have also evolved structural and sequence-based strategies to prevent aggregation. This review describes these strategies and the selection pressures that exist on protein sequences to combat their uncontrolled aggregation. We will describe the different types of mechanism evolved by proteins that adopt different conformational states including normally folded proteins, intrinsically disordered polypeptide chains, elastomeric systems and multimodular proteins.
Figures


Similar articles
-
Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins.Ann N Y Acad Sci. 2005 Dec;1066:181-221. doi: 10.1196/annals.1363.030. Ann N Y Acad Sci. 2005. PMID: 16533927 Review.
-
Partially folded intermediates as critical precursors of light chain amyloid fibrils and amorphous aggregates.Biochemistry. 2001 Mar 27;40(12):3525-35. doi: 10.1021/bi001782b. Biochemistry. 2001. PMID: 11297418
-
Proline and glycine control protein self-organization into elastomeric or amyloid fibrils.Structure. 2006 Nov;14(11):1667-76. doi: 10.1016/j.str.2006.09.008. Structure. 2006. PMID: 17098192
-
Structure analysis of an amyloid-forming model peptide by a systematic glycine and proline scan.Biomacromolecules. 2011 Aug 8;12(8):2988-96. doi: 10.1021/bm200587m. Epub 2011 Jul 13. Biomacromolecules. 2011. PMID: 21726080
-
Protein misfolding, aggregation and mechanism of amyloid cytotoxicity: An overview and therapeutic strategies to inhibit aggregation.Int J Biol Macromol. 2019 Aug 1;134:1022-1037. doi: 10.1016/j.ijbiomac.2019.05.109. Epub 2019 May 22. Int J Biol Macromol. 2019. PMID: 31128177 Review.
Cited by
-
Protein aggregation profile of the human kinome.Front Physiol. 2012 Nov 20;3:438. doi: 10.3389/fphys.2012.00438. eCollection 2012. Front Physiol. 2012. PMID: 23181023 Free PMC article.
-
Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles.Mol Cell. 2015 Mar 5;57(5):936-947. doi: 10.1016/j.molcel.2015.01.013. Mol Cell. 2015. PMID: 25747659 Free PMC article.
-
Biochemical nature of Russell Bodies.Sci Rep. 2015 Jul 30;5:12585. doi: 10.1038/srep12585. Sci Rep. 2015. PMID: 26223695 Free PMC article.
-
Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers.Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12479-84. doi: 10.1073/pnas.1117799109. Epub 2012 Jul 16. Proc Natl Acad Sci U S A. 2012. PMID: 22802614 Free PMC article.
-
Ovalbumin self-assembles into amyloid nanosheets that elicit immune responses and facilitate sustained drug release.J Biol Chem. 2018 Jul 20;293(29):11310-11324. doi: 10.1074/jbc.RA118.002550. Epub 2018 May 31. J Biol Chem. 2018. PMID: 29853634 Free PMC article.
References
-
- Bastolla U, Demetrius L (2005) Stability constraints and protein evolution: the role of chain length, composition and disulfide bonds. Protein Eng Des Sel 18: 405–415 - PubMed
-
- Bastolla U, Moya A, Viguera E, van Ham RC (2004) Genomic determinants of protein folding thermodynamics in prokaryotic organisms. J Mol Biol 343: 1451–1466 - PubMed
-
- Broome BM, Hecht MH (2000) Nature disfavors sequences of alternating polar and non-polar amino acids: implications for amyloidogenesis. J Mol Biol 296: 961–968 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

