Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment
- PMID: 17664130
- PMCID: PMC2031860
- DOI: 10.1016/j.freeradbiomed.2007.05.037
Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment
Abstract
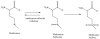
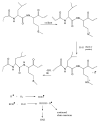
Oxidative stress has been implicated to play a crucial role in the pathogenesis of a number of diseases, including neurodegenerative disorders, cancer, and ischemia, just to name a few. Alzheimer disease (AD) is an age-related neurodegenerative disorder that is recognized as the most common form of dementia. AD is histopathologically characterized by the presence of extracellular amyloid plaques, intracellular neurofibrillary tangles, the presence of oligomers of amyloid beta-peptide (Abeta), and synapse loss. In this review we discuss the role of Abeta in the pathogenesis of AD and also the use of redox proteomics to identify oxidatively modified brain proteins in AD and mild cognitive impairment. In addition, redox proteomics studies in in vivo models of AD centered around human Abeta(1-42) are discussed.
Figures



Similar articles
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment.J Bioenerg Biomembr. 2009 Oct;41(5):441-6. doi: 10.1007/s10863-009-9241-7. J Bioenerg Biomembr. 2009. PMID: 19777328 Free PMC article. Review.
-
Redox proteomics and amyloid β-peptide: insights into Alzheimer disease.J Neurochem. 2019 Nov;151(4):459-487. doi: 10.1111/jnc.14589. Epub 2018 Nov 27. J Neurochem. 2019. PMID: 30216447 Free PMC article. Review.
-
Oxidatively modified proteins in Alzheimer's disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis.Acta Neuropathol. 2009 Jul;118(1):131-50. doi: 10.1007/s00401-009-0517-0. Epub 2009 Mar 14. Acta Neuropathol. 2009. PMID: 19288120 Free PMC article. Review.
-
Redox proteomics identification of oxidatively modified proteins in Alzheimer's disease brain and in vivo and in vitro models of AD centered around Abeta(1-42).J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Mar 20;833(1):3-11. doi: 10.1016/j.jchromb.2005.09.024. Epub 2005 Oct 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2006. PMID: 16236561 Review.
Cited by
-
Lack of p53 affects the expression of several brain mitochondrial proteins: insights from proteomics into important pathways regulated by p53.PLoS One. 2012;7(11):e49846. doi: 10.1371/journal.pone.0049846. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209608 Free PMC article.
-
Telomere shortening and Alzheimer's disease.Neuromolecular Med. 2013 Mar;15(1):25-48. doi: 10.1007/s12017-012-8207-9. Epub 2012 Nov 16. Neuromolecular Med. 2013. PMID: 23161153 Review.
-
Renal Contributions in the Pathophysiology and Neuropathological Substrates Shared by Chronic Kidney Disease and Alzheimer's Disease.Brain Sci. 2020 Aug 17;10(8):563. doi: 10.3390/brainsci10080563. Brain Sci. 2020. PMID: 32824404 Free PMC article. Review.
-
Action of trichostatin A on Alzheimer's disease-like pathological changes in SH-SY5Y neuroblastoma cells.Neural Regen Res. 2020 Feb;15(2):293-301. doi: 10.4103/1673-5374.265564. Neural Regen Res. 2020. PMID: 31552902 Free PMC article.
-
Diastereomeric Mixture of Calophyllic and Isocalophyllic Acid Ameliorates Scopolamine-Induced Memory Impairment in Mice: Involvement of Antioxidant Defense and Cholinergic Systems.Neurotox Res. 2020 Jan;37(1):58-66. doi: 10.1007/s12640-019-00117-8. Epub 2019 Oct 26. Neurotox Res. 2020. PMID: 31656017
References
-
- Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002;36:1307–1313. - PubMed
-
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. - PubMed
-
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. - PubMed
-
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. - PubMed
-
- Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010836-130011/AG/NIA NIH HHS/United States
- P01 AG005119-110001/AG/NIA NIH HHS/United States
- P01 AG010836-120011/AG/NIA NIH HHS/United States
- P01 AG005119-140001/AG/NIA NIH HHS/United States
- P01 AG005119/AG/NIA NIH HHS/United States
- P01 AG005119-130001/AG/NIA NIH HHS/United States
- AG-05119/AG/NIA NIH HHS/United States
- P01 AG010836/AG/NIA NIH HHS/United States
- P01 AG005119-120001/AG/NIA NIH HHS/United States
- P01 AG005119-13S10001/AG/NIA NIH HHS/United States
- P01 AG005119-150006/AG/NIA NIH HHS/United States
- P01 AG010836-110011/AG/NIA NIH HHS/United States
- P01 AG010836-140011/AG/NIA NIH HHS/United States
- P01 AG005119-12S20001/AG/NIA NIH HHS/United States
- AG-10836/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

