Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta
- PMID: 17655740
- PMCID: PMC2433297
- DOI: 10.1111/j.1365-2567.2007.02687.x
Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta
Abstract
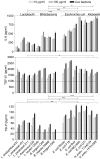
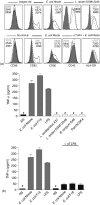
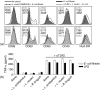
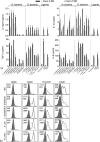
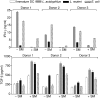
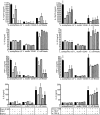
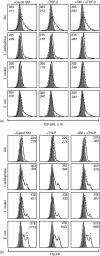
Humans and other mammals coexist with a diverse array of microbes colonizing the intestine, termed the microflora. The relationship is symbiotic, with the microbes benefiting from a stable environment and nutrient supply, and the host gaining competitive exclusion of pathogens and continuously maintenance of the gut immune homeostasis. Here we report novel crosstalk mechanisms between the human enterocyte cell line, Caco2, and underlying human monocyte-derived DC in a transwell model where Gram-positive (G+) commensals prevent Toll-like receptor-4 (TLR4)-dependent Escherichia coli-induced semimaturation in a TLR2-dependent fashion. These findings add to our understanding of the hypo-responsiveness of the gut epithelium towards the microflora. Gut DC posses a more tolerogenic phenotype than conventional DC. Here we show that Caco2 spent medium (SM) induces tolerogenic DC with lower expression of maturation markers, interleukin (IL)-12p70, and tumour necrosis factor-alpha when matured with G+ and Gram-negative (G-) commensals, while IL-10 production is enhanced in DC upon encountering G+ commensals and reduced upon encountering G- bacteria. The Caco2 SM-induced tolerogenic phenotype is also seen in DC priming of naive T cells with elevated levels of transforming growth factor-beta (TGF-beta) and markedly reduced levels of bacteria-induced interferon-gamma production. Caco2 cell production of IL-8, thymic stromal lymphopoietin (TSLP) and TGF-beta increases upon microbial stimulation in a strain dependent manner. TSLP and TGF-beta co-operate in inducing the tolerogenic DC phenotype but other mediators might be involved.
Figures
Similar articles
-
ATP conditions intestinal epithelial cells to an inflammatory state that promotes components of DC maturation.Eur J Immunol. 2012 Dec;42(12):3310-21. doi: 10.1002/eji.201142213. Epub 2012 Oct 26. Eur J Immunol. 2012. PMID: 22987503
-
Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning.Mucosal Immunol. 2009 Jul;2(4):340-50. doi: 10.1038/mi.2009.13. Epub 2009 Apr 22. Mucosal Immunol. 2009. PMID: 19387433
-
Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells.Infect Immun. 2004 May;72(5):2671-8. doi: 10.1128/IAI.72.5.2671-2678.2004. Infect Immun. 2004. PMID: 15102775 Free PMC article.
-
Transforming growth factor and intestinal inflammation: the role of nutrition.Nestle Nutr Inst Workshop Ser. 2013;77:91-8. doi: 10.1159/000351390. Epub 2013 Aug 29. Nestle Nutr Inst Workshop Ser. 2013. PMID: 24107499 Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Functions of Dendritic Cells and Its Association with Intestinal Diseases.Cells. 2021 Mar 6;10(3):583. doi: 10.3390/cells10030583. Cells. 2021. PMID: 33800865 Free PMC article. Review.
-
Disruption of the Hedgehog signaling pathway in inflammatory bowel disease fosters chronic intestinal inflammation.Clin Exp Med. 2017 Aug;17(3):351-369. doi: 10.1007/s10238-016-0434-1. Epub 2016 Sep 21. Clin Exp Med. 2017. PMID: 27655445
-
The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties.Microorganisms. 2024 Jan 23;12(2):234. doi: 10.3390/microorganisms12020234. Microorganisms. 2024. PMID: 38399637 Free PMC article. Review.
-
The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease.Front Nutr. 2021 Nov 25;8:759507. doi: 10.3389/fnut.2021.759507. eCollection 2021. Front Nutr. 2021. PMID: 34901112 Free PMC article. Review.
-
The Use of Probiotic Therapy to Modulate the Gut Microbiota and Dendritic Cell Responses in Inflammatory Bowel Diseases.Med Sci (Basel). 2019 Feb 22;7(2):33. doi: 10.3390/medsci7020033. Med Sci (Basel). 2019. PMID: 30813381 Free PMC article. Review.
References
-
- Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. - PubMed
-
- Niess JH, Brand S, Gu XB, et al. CX (3) CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. - PubMed
-
- Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005;206:177–89. - PubMed
-
- Zeuthen LH, Christensen HR, Frokiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with Gram-negative bacteria. Clin Vaccine Immunol. 2006;13:365–75. - PMC - PubMed
-
- Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

