Tracking virus-specific CD4+ T cells during and after acute hepatitis C virus infection
- PMID: 17653276
- PMCID: PMC1920556
- DOI: 10.1371/journal.pone.0000649
Tracking virus-specific CD4+ T cells during and after acute hepatitis C virus infection
Abstract
Background: CD4+ T cell help is critical in maintaining antiviral immune responses and such help has been shown to be sustained in acute resolving hepatitis C. In contrast, in evolving chronic hepatitis C CD4+ T cell helper responses appear to be absent or short-lived, using functional assays.
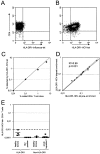
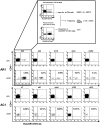
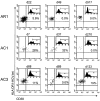
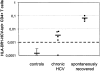
Methodology/principal findings: Here we used a novel HLA-DR1 tetramer containing a highly targeted CD4+ T cell epitope from the hepatitis C virus non-structural protein 4 to track number and phenotype of hepatitis C virus specific CD4+ T cells in a cohort of seven HLA-DR1 positive patients with acute hepatitis C in comparison to patients with chronic or resolved hepatitis C. We observed peptide-specific T cells in all seven patients with acute hepatitis C regardless of outcome at frequencies up to 0.65% of CD4+ T cells. Among patients who transiently controlled virus replication we observed loss of function, and/or physical deletion of tetramer+ CD4+ T cells before viral recrudescence. In some patients with chronic hepatitis C very low numbers of tetramer+ cells were detectable in peripheral blood, compared to robust responses detected in spontaneous resolvers. Importantly we did not observe escape mutations in this key CD4+ T cell epitope in patients with evolving chronic hepatitis C.
Conclusions/significance: During acute hepatitis C a CD4+ T cell response against this epitope is readily induced in most, if not all, HLA-DR1+ patients. This antiviral T cell population becomes functionally impaired or is deleted early in the course of disease in those where viremia persists.
Conflict of interest statement
Figures


Similar articles
-
Conserved hierarchy of helper T cell responses in a chimpanzee during primary and secondary hepatitis C virus infections.J Immunol. 2004 Jan 1;172(1):483-92. doi: 10.4049/jimmunol.172.1.483. J Immunol. 2004. PMID: 14688358
-
Identification of novel HLA-DR1-restricted epitopes from the hepatitis B virus envelope protein in mice expressing HLA-DR1 and vaccinated human subjects.Microbes Infect. 2006 Oct;8(12-13):2783-90. doi: 10.1016/j.micinf.2006.08.009. Epub 2006 Sep 12. Microbes Infect. 2006. PMID: 17045504
-
Virus-Specific CD4+ T Cells Have Functional and Phenotypic Characteristics of Follicular T-Helper Cells in Patients With Acute and Chronic HCV Infections.Gastroenterology. 2016 Mar;150(3):696-706.e3. doi: 10.1053/j.gastro.2015.11.005. Epub 2015 Nov 14. Gastroenterology. 2016. PMID: 26584604
-
The role of hepatitis C virus specific CD4+ T lymphocytes in acute and chronic hepatitis C.J Mol Med (Berl). 1996 Oct;74(10):583-8. doi: 10.1007/s001090050062. J Mol Med (Berl). 1996. PMID: 8912179 Review.
-
CD4+ T cell responses in hepatitis C virus infection.World J Gastroenterol. 2007 Sep 28;13(36):4831-8. doi: 10.3748/wjg.v13.i36.4831. World J Gastroenterol. 2007. PMID: 17828814 Free PMC article. Review.
Cited by
-
A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans.PLoS One. 2013;8(3):e59776. doi: 10.1371/journal.pone.0059776. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23527266 Free PMC article.
-
Adaptive Immune Response against Hepatitis C Virus.Int J Mol Sci. 2020 Aug 6;21(16):5644. doi: 10.3390/ijms21165644. Int J Mol Sci. 2020. PMID: 32781731 Free PMC article. Review.
-
Evidence of CD4+ T cell-mediated immune pressure on the Hepatitis C virus genome.Sci Rep. 2018 May 8;8(1):7224. doi: 10.1038/s41598-018-25559-6. Sci Rep. 2018. PMID: 29740042 Free PMC article.
-
Direct ex-vivo evaluation of pneumococcal specific T-cells in healthy adults.PLoS One. 2011;6(10):e25367. doi: 10.1371/journal.pone.0025367. Epub 2011 Oct 24. PLoS One. 2011. PMID: 22039412 Free PMC article.
-
CD4+ T Cell Differentiation in Chronic Viral Infections: The Tfh Perspective.Trends Mol Med. 2017 Dec;23(12):1072-1087. doi: 10.1016/j.molmed.2017.10.001. Epub 2017 Nov 12. Trends Mol Med. 2017. PMID: 29137933 Free PMC article. Review.
References
-
- Butt AA. Hepatitis C virus infection: the new global epidemic. Expert Rev Anti Infect Ther. 2005;3:241–249. - PubMed
-
- Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

