Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle
- PMID: 17645733
- PMCID: PMC2967192
- DOI: 10.1111/j.1365-2958.2007.05860.x
Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle
Abstract
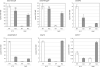
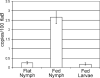
Borrelia burgdorferi (Bb) adapts to its arthropod and mammalian hosts by altering its transcriptional and antigenic profiles in response to environmental signals associated with each of these milieus. In studies presented here, we provide evidence to suggest that mammalian host signals are important for modulating and maintaining both the positive and negative aspects of mammalian host adaptation mediated by the alternative sigma factor RpoS in Bb. Although considerable overlap was observed between genes induced by RpoS during growth within the mammalian host and following temperature-shift, comparative microarray analyses demonstrated unequivocally that RpoS-mediated repression requires mammalian host-specific signals. A substantial portion of the in vivo RpoS regulon was uniquely upregulated within dialysis membrane chambers, further underscoring the importance of host-derived environmental stimuli for differential gene expression in Bb. Expression profiling of genes within the RpoS regulon by quantitative reverse transcription polymerase chain reaction (qRT-PCR) revealed a level of complexity to RpoS-dependent gene regulation beyond that observed by microarray, including a broad range of expression levels and the presence of genes whose expression is only partially dependent on RpoS. Analysis of Bb-infected ticks by qRT-PCR established that expression of rpoS is induced during the nymphal blood meal but not within unfed nymphs or engorged larvae. Together, these data have led us to postulate that RpoS acts as a gatekeeper for the reciprocal regulation of genes involved in the establishment of infection within the mammalian host and the maintenance of spirochetes within the arthropod vector.
Figures
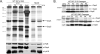

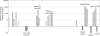


Similar articles
-
BosR and PlzA reciprocally regulate RpoS function to sustain Borrelia burgdorferi in ticks and mammals.J Clin Invest. 2023 Mar 1;133(5):e166710. doi: 10.1172/JCI166710. J Clin Invest. 2023. PMID: 36649080 Free PMC article.
-
Two Distinct Mechanisms Govern RpoS-Mediated Repression of Tick-Phase Genes during Mammalian Host Adaptation by Borrelia burgdorferi, the Lyme Disease Spirochete.mBio. 2017 Aug 22;8(4):e01204-17. doi: 10.1128/mBio.01204-17. mBio. 2017. PMID: 28830947 Free PMC article.
-
RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants.Infect Immun. 2004 Nov;72(11):6433-45. doi: 10.1128/IAI.72.11.6433-6445.2004. Infect Immun. 2004. PMID: 15501774 Free PMC article.
-
Gene regulation in Borrelia burgdorferi.Annu Rev Microbiol. 2011;65:479-99. doi: 10.1146/annurev.micro.112408.134040. Annu Rev Microbiol. 2011. PMID: 21801026 Review.
-
Adaptation of Borrelia burgdorferi in the vector and vertebrate host.Microbes Infect. 2003 Jun;5(7):659-66. doi: 10.1016/s1286-4579(03)00097-2. Microbes Infect. 2003. PMID: 12787742 Review.
Cited by
-
Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector.Future Microbiol. 2013 Jan;8(1):41-56. doi: 10.2217/fmb.12.121. Future Microbiol. 2013. PMID: 23252492 Free PMC article. Review.
-
Characterization of the RelBbu Regulon in Borrelia burgdorferi Reveals Modulation of Glycerol Metabolism by (p)ppGpp.PLoS One. 2015 Feb 17;10(2):e0118063. doi: 10.1371/journal.pone.0118063. eCollection 2015. PLoS One. 2015. PMID: 25688856 Free PMC article.
-
Characterization of a conditional bosR mutant in Borrelia burgdorferi.Infect Immun. 2010 Jan;78(1):265-74. doi: 10.1128/IAI.01018-09. Epub 2009 Oct 26. Infect Immun. 2010. PMID: 19858309 Free PMC article.
-
BosR and PlzA reciprocally regulate RpoS function to sustain Borrelia burgdorferi in ticks and mammals.J Clin Invest. 2023 Mar 1;133(5):e166710. doi: 10.1172/JCI166710. J Clin Invest. 2023. PMID: 36649080 Free PMC article.
-
Membrane directed expression in Escherichia coli of BBA57 and other virulence factors from the Lyme disease agent Borrelia burgdorferi.Sci Rep. 2019 Nov 26;9(1):17606. doi: 10.1038/s41598-019-53830-x. Sci Rep. 2019. PMID: 31772280 Free PMC article.
References
-
- Akins DR, Porcella SF, Popova TG, Shevchenko D, Baker SI, Li M, et al. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homolog. Mol Microbiol. 1995;18:507–520. - PubMed
-
- Alverson J, Bundle SF, Sohaskey CD, Lybecker MC, Samuels DS. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol Microbiol. 2003;48:1665–1677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

