Regulation of NMDA receptors by phosphorylation
- PMID: 17644144
- PMCID: PMC2001266
- DOI: 10.1016/j.neuropharm.2007.05.018
Regulation of NMDA receptors by phosphorylation
Abstract
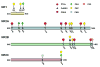
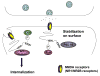
N-methyl-D-aspartate (NMDA) receptors are critical for neuronal development and synaptic plasticity. The molecular mechanisms underlying the synaptic localization and functional regulation of NMDA receptors have been the subject of extensive studies. In particular, phosphorylation has emerged as a fundamental mechanism that regulates NMDA receptor trafficking and can alter the channel properties of NMDA receptors. Here we summarize recent advances in the characterization of NMDA receptor phosphorylation, emphasizing subunit-specific phosphorylation, which differentially controls the trafficking and surface expression of NMDA receptors.
Figures
Similar articles
-
NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity.Trends Neurosci. 2002 Nov;25(11):571-7. doi: 10.1016/s0166-2236(02)02272-5. Trends Neurosci. 2002. PMID: 12392932 Review.
-
Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis.Brain. 2012 May;135(Pt 5):1606-21. doi: 10.1093/brain/aws092. Brain. 2012. PMID: 22544902
-
Single amino acid residue in the M4 domain of GluN1 subunit regulates the surface delivery of NMDA receptors.J Neurochem. 2012 Nov;123(3):385-95. doi: 10.1111/jnc.12002. Epub 2012 Sep 28. J Neurochem. 2012. PMID: 22937865
-
Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand.J Neurosci. 2004 Nov 10;24(45):10248-59. doi: 10.1523/JNEUROSCI.0546-04.2004. J Neurosci. 2004. PMID: 15537897 Free PMC article.
-
NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders.Nat Rev Neurosci. 2007 Jun;8(6):413-26. doi: 10.1038/nrn2153. Nat Rev Neurosci. 2007. PMID: 17514195 Review.
Cited by
-
Effects of Combined Bushen Zhichan Recipe and Levodopa in a Rodent Model of Parkinson Disease: Potential Mechanisms.Med Sci Monit. 2020 Jun 19;26:e922345. doi: 10.12659/MSM.922345. Med Sci Monit. 2020. PMID: 32555131 Free PMC article.
-
Local modulation of steroid action: rapid control of enzymatic activity.Front Neurosci. 2015 Mar 19;9:83. doi: 10.3389/fnins.2015.00083. eCollection 2015. Front Neurosci. 2015. PMID: 25852459 Free PMC article. Review.
-
Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors.J Neurosci. 2013 Feb 27;33(9):4151-64. doi: 10.1523/JNEUROSCI.2721-12.2013. J Neurosci. 2013. PMID: 23447623 Free PMC article.
-
Ionotropic glutamate receptors: regulation by G-protein-coupled receptors.Mol Pharmacol. 2013 Apr;83(4):746-52. doi: 10.1124/mol.112.083352. Epub 2013 Jan 24. Mol Pharmacol. 2013. PMID: 23348498 Free PMC article. Review.
-
The Glutamate System as a Crucial Regulator of CNS Toxicity and Survival of HIV Reservoirs.Front Cell Infect Microbiol. 2020 Jun 24;10:261. doi: 10.3389/fcimb.2020.00261. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32670889 Free PMC article. Review.
References
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. - PubMed
-
- Chen BS, Braud S, Badger JD, 2nd, Isaac JT, Roche KW. Regulation of NR1/NR2C N-methyl-D-aspartate (NMDA) receptors by phosphorylation. The Journal of biological chemistry. 2006;281:16583–16590. - PubMed
-
- Chen BS, Isaac JT, Roche KW. PKB Phosphorylation of the NMDA Receptor Subunit NR2C and Binding to 14-3-3ε. Society for Neuroscience 35th Annual Meeting Abstract 844.9 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

