Trk-signaling endosomes are generated by Rac-dependent macroendocytosis
- PMID: 17640889
- PMCID: PMC1941461
- DOI: 10.1073/pnas.0702819104
Trk-signaling endosomes are generated by Rac-dependent macroendocytosis
Abstract
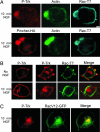
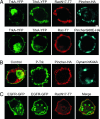
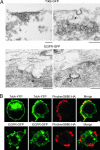
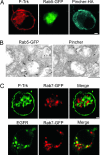
Why neurotrophins and their Trk receptors promote neuronal differentiation and survival whereas receptor tyrosine kinases for other growth factors, such as EGF, do not, has been a long-standing question in neurobiology. We provide evidence that one difference lies in the selective ability of Trk to generate long-lived signaling endosomes. We show that Trk endocytosis is distinguished from the classical clathrin-based endocytosis of EGF receptor (EGFR). Although Trk and EGFR each stimulate membrane ruffling, only Trk undergoes both selective and specific macroendocytosis at ruffles, which uniquely requires the Rho-GTPase, Rac, and the trafficking protein, Pincher. This process leads to Trk-signaling endosomes, which are immature multivesicular bodies that retain Rab5. In contrast, EGFR endosomes rapidly exchange Rab5 for Rab7, thereby transiting into late-endosomes/lysosomes for degradation. Sustained endosomal signaling by Trk does not reflect intrinsic differences between Trk and EGFR, because each elicits long-term Erk-kinase activation from the cell surface. Thus, a population of stable Trk endosomes, formed by specialized macroendocytosis in neurons, provides a privileged endosome-based system for propagation of signals to the nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors.Traffic. 2011 Sep;12(9):1099-108. doi: 10.1111/j.1600-0854.2011.01213.x. Epub 2011 May 23. Traffic. 2011. PMID: 21535338 Free PMC article. Review.
-
Trk retrograde signaling requires persistent, Pincher-directed endosomes.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):852-7. doi: 10.1073/pnas.1015981108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187387 Free PMC article.
-
Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors.J Neurosci. 2005 May 25;25(21):5236-47. doi: 10.1523/JNEUROSCI.5104-04.2005. J Neurosci. 2005. PMID: 15917464 Free PMC article.
-
Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes.J Cell Biol. 2002 May 13;157(4):679-91. doi: 10.1083/jcb.200201063. Epub 2002 May 13. J Cell Biol. 2002. PMID: 12011113 Free PMC article.
-
Retrograde signaling by the neurotrophins follows a well-worn trk.Trends Neurosci. 2002 Aug;25(8):379-81. doi: 10.1016/s0166-2236(02)02199-9. Trends Neurosci. 2002. PMID: 12127743 Review.
Cited by
-
Retrograde neurotrophic signaling requires a protein interacting with receptor tyrosine kinases via C2H2 zinc fingers.Mol Biol Cell. 2010 Jan 1;21(1):36-49. doi: 10.1091/mbc.e09-04-0321. Epub 2009 Oct 28. Mol Biol Cell. 2010. PMID: 19864463 Free PMC article.
-
Endocytosis and endosomes at the crossroads of regulating trafficking of axon outgrowth-modifying receptors.Traffic. 2011 Sep;12(9):1099-108. doi: 10.1111/j.1600-0854.2011.01213.x. Epub 2011 May 23. Traffic. 2011. PMID: 21535338 Free PMC article. Review.
-
A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes.J Cell Biol. 2009 Mar 23;184(6):863-79. doi: 10.1083/jcb.200807186. Epub 2009 Mar 16. J Cell Biol. 2009. PMID: 19289794 Free PMC article.
-
Trk retrograde signaling requires persistent, Pincher-directed endosomes.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):852-7. doi: 10.1073/pnas.1015981108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187387 Free PMC article.
-
Regulation of neurotrophin receptor (Trk) signaling: suppressor of cytokine signaling 2 (SOCS2) is a new player.Front Mol Neurosci. 2014 May 14;7:39. doi: 10.3389/fnmol.2014.00039. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24860421 Free PMC article. Review.
References
-
- Halegoua S, Armstrong RC, Kremer NE. Curr Top Microbiol Immunol. 1991;165:119–170. - PubMed
-
- Qiu MS, Green SH. Neuron. 1991;7:937–946. - PubMed
-
- Thomas SM, DeMarco M, D'Arcangelo G, Halegoua S, Brugge JS. Cell. 1992;68:1031–1040. - PubMed
-
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Nature. 1998;392:622–626. - PubMed
-
- Cowley S, Paterson H, Kemp P, Marshall CJ. Cell. 1994;77:841–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

