Absence of gemin5 from SMN complexes in nuclear Cajal bodies
- PMID: 17640370
- PMCID: PMC1939999
- DOI: 10.1186/1471-2121-8-28
Absence of gemin5 from SMN complexes in nuclear Cajal bodies
Abstract
Background: Spinal muscular atrophy is caused by reduced levels of the survival of motor neurons (SMN) protein. SMN is found in large complexes with Sm proteins and at least eight other proteins, including seven "gemins". These complexes are involved in the assembly of snRNPs in the cytoplasm and their transport into the nucleus, but the precise roles of the individual protein components are largely unknown.
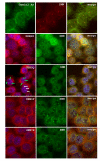
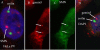
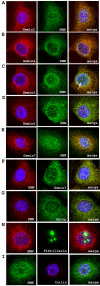
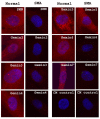
Results: We have investigated the subcellular distribution of gemins using novel antibodies against gemins 3-7, and existing mAbs against SMN, gemin2, unrip, fibrillarin and profilin II. Most gemins were equally distributed between nuclear and cytoplasmic fractions of HeLa cells, but gemin5 and unrip were more abundant in the cytoplasm. In a cytoplasmic extract obtained by mild disruption of HeLa cells, nearly all the SMN and gemins 2-4 were in large complexes, but most of the gemin5 sedimented separately with a lower S value. Most of the unrip sedimented with gemins 6 and 7 near the top of the sucrose density gradients, separate from both SMN and gemin5. Anti-SMN mAbs pulled down gemin5 from cytoplasmic extracts, but not from nuclear extracts, and gemin5 did not co-sediment with large SMN complexes in nuclear extracts. These data suggest that gemin5 is easily detached from SMN-gemin complexes in the nucleus. By immuno-histochemistry, gemin5 was rarely detectable in nuclear gems/Cajal bodies, although it was accessible to antibody and easily detectable when present. This suggests that gemin5 is normally absent from SMN complexes in these nuclear storage sites.
Conclusion: We conclude that SMN complexes usually exist without gemin5 in nuclear gems/Cajal bodies. Gemin5 is believed to be involved in capturing snRNA into SMN complexes in the cytoplasm for transport into the nucleus. We hypothesize that gemin5, though present in the nucleus, is no longer needed for SMN complex function during the time these complexes are stored in gems/Cajal bodies.
Figures





Similar articles
-
Unrip, a factor implicated in cap-independent translation, associates with the cytosolic SMN complex and influences its intracellular localization.Hum Mol Genet. 2005 Oct 15;14(20):3099-111. doi: 10.1093/hmg/ddi343. Epub 2005 Sep 13. Hum Mol Genet. 2005. PMID: 16159890
-
Analysis of SMN-neurite granules: Core Cajal body components are absent from SMN-cytoplasmic complexes.Biochem Biophys Res Commun. 2010 Jul 2;397(3):479-85. doi: 10.1016/j.bbrc.2010.05.139. Epub 2010 May 31. Biochem Biophys Res Commun. 2010. PMID: 20515655
-
A role for complexes of survival of motor neurons (SMN) protein with gemins and profilin in neurite-like cytoplasmic extensions of cultured nerve cells.Exp Cell Res. 2005 Sep 10;309(1):185-97. doi: 10.1016/j.yexcr.2005.05.014. Exp Cell Res. 2005. PMID: 15975577
-
SMN and Gemins: 'we are family' … or are we?: insights into the partnership between Gemins and the spinal muscular atrophy disease protein SMN.Bioessays. 2010 Dec;32(12):1077-89. doi: 10.1002/bies.201000088. Epub 2010 Oct 15. Bioessays. 2010. PMID: 20954180 Review.
-
SMN - A chaperone for nuclear RNP social occasions?RNA Biol. 2017 Jun 3;14(6):701-711. doi: 10.1080/15476286.2016.1236168. Epub 2016 Sep 20. RNA Biol. 2017. PMID: 27648855 Free PMC article. Review.
Cited by
-
Genetic Interactions between the Members of the SMN-Gemins Complex in Drosophila.PLoS One. 2015 Jun 22;10(6):e0130974. doi: 10.1371/journal.pone.0130974. eCollection 2015. PLoS One. 2015. PMID: 26098872 Free PMC article.
-
A novel functional role for MMSET in RNA processing based on the link between the REIIBP isoform and its interaction with the SMN complex.PLoS One. 2014 Jun 12;9(6):e99493. doi: 10.1371/journal.pone.0099493. eCollection 2014. PLoS One. 2014. PMID: 24923560 Free PMC article.
-
Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy.Hum Mol Genet. 2008 Aug 15;17(16):2552-69. doi: 10.1093/hmg/ddn156. Epub 2008 May 20. Hum Mol Genet. 2008. PMID: 18492800 Free PMC article.
-
Gemin5 promotes IRES interaction and translation control through its C-terminal region.Nucleic Acids Res. 2013 Jan;41(2):1017-28. doi: 10.1093/nar/gks1212. Epub 2012 Dec 5. Nucleic Acids Res. 2013. PMID: 23221641 Free PMC article.
-
Gemin5: A Multitasking RNA-Binding Protein Involved in Translation Control.Biomolecules. 2015 Apr 17;5(2):528-44. doi: 10.3390/biom5020528. Biomolecules. 2015. PMID: 25898402 Free PMC article. Review.
References
-
- Gubitz AK, Feng W, Dreyfuss G. The SMN complex. Exp Cell Res. 2004;296:51–56. - PubMed
-
- Otter S, Grimmler M, Neuenkirchen N, Chari A, Sickmann A, Fischer U. A Comprehensive Interaction Map of the Human Survival of Motor Neuron (SMN) Complex. J Biol Chem. 2007;282:5825–5833. - PubMed
-
- Baccon J, Pellizzoni L, Rappsilber J, Mann M, Dreyfuss G. Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J Biol Chem. 2002;277:31957–31962. - PubMed
-
- Carissimi C, Saieva L, Gabanella F, Pellizzoni L. Gemin8 is required for the architecture and function of the survival motor neuron complex. J Biol Chem. 2006;281:37009–37016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

