The membrane attack complex of the complement system is essential for rapid Wallerian degeneration
- PMID: 17634361
- PMCID: PMC6672891
- DOI: 10.1523/JNEUROSCI.5623-06.2007
The membrane attack complex of the complement system is essential for rapid Wallerian degeneration
Abstract
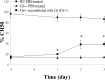
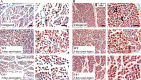
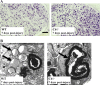
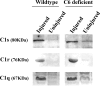
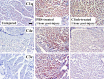
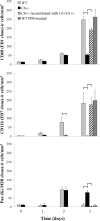
The complement (C) system plays an important role in myelin breakdown during Wallerian degeneration (WD). The pathway and mechanism involved are, however, not clear. In a crush injury model of the sciatic nerve, we show that C6, necessary for the assembly of the membrane attack complex (MAC), is essential for rapid WD. At 3 d after injury, pronounced WD occurred in wild-type animals, whereas the axons and myelin of C6-deficient animals appeared intact. Macrophage recruitment and activation was inhibited in C6-deficient rats. However, 7 d after injury, the distal part of the C6-deficient nerves appeared degraded. As a consequence of a delayed WD, more myelin breakdown products were present than in wild-type nerves. Reconstitution of the C6-deficient animals with C6 restored the wild-type phenotype. Treatment with rhC1INH (recombinant human complement 1 inhibitor) blocked deposition of activated C-cleaved products after injury. These experiments demonstrate that the classical pathway of the complement system is activated after acute nerve trauma and that the entire complement cascade, including MAC deposition, is essential for rapid WD and efficient clearance of myelin after acute peripheral nerve trauma.
Figures



Similar articles
-
Local production of serum amyloid a is implicated in the induction of macrophage chemoattractants in Schwann cells during wallerian degeneration of peripheral nerves.Glia. 2012 Oct;60(10):1619-28. doi: 10.1002/glia.22382. Epub 2012 Jul 9. Glia. 2012. PMID: 22777957
-
C6 deficiency does not alter intrinsic regeneration speed after peripheral nerve crush injury.Neurosci Res. 2014 Oct;87:26-32. doi: 10.1016/j.neures.2014.06.008. Epub 2014 Jul 7. Neurosci Res. 2014. PMID: 25011063
-
TGF-β1 is critical for Wallerian degeneration after rat sciatic nerve injury.Neuroscience. 2015 Jan 22;284:759-767. doi: 10.1016/j.neuroscience.2014.10.051. Epub 2014 Nov 4. Neuroscience. 2015. PMID: 25451291
-
Membrane attack complex of complement is not essential for immune mediated demyelination in experimental autoimmune neuritis.J Neuroimmunol. 2010 Dec 15;229(1-2):98-106. doi: 10.1016/j.jneuroim.2010.07.010. Epub 2010 Sep 17. J Neuroimmunol. 2010. PMID: 20850187
-
Differential expression and potential role of SOCS1 and SOCS3 in Wallerian degeneration in injured peripheral nerve.Exp Neurol. 2010 May;223(1):173-82. doi: 10.1016/j.expneurol.2009.06.018. Epub 2009 Jul 2. Exp Neurol. 2010. PMID: 19576891 Free PMC article.
Cited by
-
Generation of 1E8 Single Chain Fv-Fc Construct Against Human CD59.Immune Netw. 2012 Feb;12(1):33-9. doi: 10.4110/in.2012.12.1.33. Epub 2012 Feb 29. Immune Netw. 2012. PMID: 22536168 Free PMC article.
-
Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice.J Neuroinflammation. 2013 Jun 27;10:76. doi: 10.1186/1742-2094-10-76. J Neuroinflammation. 2013. PMID: 23806181 Free PMC article.
-
Wallerian degeneration, wld(s), and nmnat.Annu Rev Neurosci. 2010;33:245-67. doi: 10.1146/annurev-neuro-060909-153248. Annu Rev Neurosci. 2010. PMID: 20345246 Free PMC article. Review.
-
Systemic inhibition of the membrane attack complex impedes neuroinflammation in chronic relapsing experimental autoimmune encephalomyelitis.Acta Neuropathol Commun. 2018 May 3;6(1):36. doi: 10.1186/s40478-018-0536-y. Acta Neuropathol Commun. 2018. PMID: 29724241 Free PMC article.
-
Microbial Neuraminidase Induces a Moderate and Transient Myelin Vacuolation Independent of Complement System Activation.Front Neurol. 2017 Mar 7;8:78. doi: 10.3389/fneur.2017.00078. eCollection 2017. Front Neurol. 2017. PMID: 28326060 Free PMC article.
References
-
- Ballin RH, Thomas PK. Changes at the nodes of Ranvier during Wallerian degeneration: an electron microscope study. Acta Neuropathol (Berl) 1969;14:237–249. - PubMed
-
- Barnum SR, Szalai AJ. Complement and demyelinating disease: no MAC needed? Brain Res Brain Res Rev. 2006;52:58–68. - PubMed
-
- Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. - PubMed
-
- Bhole D, Stahl GL. Molecular basis for complement component 6 (C6) deficiency in rats and mice. Immunobiology. 2004;209:559–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
