Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy
- PMID: 17632570
- PMCID: PMC7098186
- DOI: 10.1038/nrmicro1707
Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy
Abstract
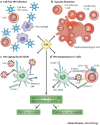
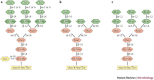
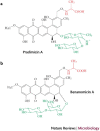
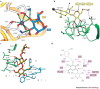
Several chronic viral infections (such as HIV and hepatitis C virus) are highly prevalent and are a serious health risk. The adaptation of animal viruses to the human host, as recently exemplified by influenza viruses and the severe acute respiratory syndrome coronavirus, is also a continuous threat. There is a high demand, therefore, for new antiviral lead compounds and novel therapeutic concepts. In this Review, an original therapeutic concept for suppressing enveloped viruses is presented that is based on a specific interaction of carbohydrate-binding agents (CBAs) with the glycans present on viral-envelope glycoproteins. This approach may also be extended to other pathogens, including parasites, bacteria and fungi.
Conflict of interest statement
The author declares no competing financial interests.
Figures
Similar articles
-
Phenylboronic-acid-based carbohydrate binders as antiviral therapeutics: monophenylboronic acids.Antivir Chem Chemother. 2010 Aug 11;20(6):249-57. doi: 10.3851/IMP1632. Antivir Chem Chemother. 2010. PMID: 20710065
-
Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV.Lancet Infect Dis. 2005 Nov;5(11):726-31. doi: 10.1016/S1473-3099(05)70271-1. Lancet Infect Dis. 2005. PMID: 16253890
-
Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses?Antivir Chem Chemother. 2007;18(1):1-11. doi: 10.1177/095632020701800101. Antivir Chem Chemother. 2007. PMID: 17354647 Review.
-
Glycerol Monolaurate, an Analogue to a Factor Secreted by Lactobacillus, Is Virucidal against Enveloped Viruses, Including HIV-1.mBio. 2020 May 5;11(3):e00686-20. doi: 10.1128/mBio.00686-20. mBio. 2020. PMID: 32371599 Free PMC article.
-
Comparison of antiviral resistance across acute and chronic viral infections.Antiviral Res. 2018 Oct;158:103-112. doi: 10.1016/j.antiviral.2018.07.020. Epub 2018 Aug 4. Antiviral Res. 2018. PMID: 30086337 Review.
Cited by
-
Algal lectins as promising biomolecules for biomedical research.Crit Rev Microbiol. 2015 Feb;41(1):77-88. doi: 10.3109/1040841X.2013.798780. Epub 2013 Jul 16. Crit Rev Microbiol. 2015. PMID: 23855360 Free PMC article. Review.
-
Combinations of griffithsin with other carbohydrate-binding agents demonstrate superior activity against HIV Type 1, HIV Type 2, and selected carbohydrate-binding agent-resistant HIV Type 1 strains.AIDS Res Hum Retroviruses. 2012 Nov;28(11):1513-23. doi: 10.1089/AID.2012.0026. Epub 2012 Jun 25. AIDS Res Hum Retroviruses. 2012. PMID: 22607556 Free PMC article.
-
Lectins with anti-HIV activity: a review.Molecules. 2015 Jan 6;20(1):648-68. doi: 10.3390/molecules20010648. Molecules. 2015. PMID: 25569520 Free PMC article. Review.
-
Structural insights into the specific anti-HIV property of actinohivin: structure of its complex with the α(1-2)mannobiose moiety of gp120.Acta Crystallogr D Biol Crystallogr. 2012 Dec;68(Pt 12):1671-9. doi: 10.1107/S0907444912040498. Epub 2012 Nov 9. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 23151632 Free PMC article.
-
The role of individual carbohydrate-binding sites in the function of the potent anti-HIV lectin griffithsin.Mol Pharm. 2012 Sep 4;9(9):2613-25. doi: 10.1021/mp300194b. Epub 2012 Aug 21. Mol Pharm. 2012. PMID: 22827601 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

