Effect of endurance exercise training on Ca2+ calmodulin-dependent protein kinase II expression and signalling in skeletal muscle of humans
- PMID: 17627985
- PMCID: PMC2277010
- DOI: 10.1113/jphysiol.2007.138529
Effect of endurance exercise training on Ca2+ calmodulin-dependent protein kinase II expression and signalling in skeletal muscle of humans
Abstract
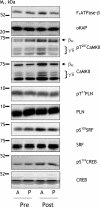
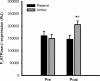
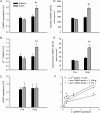
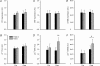
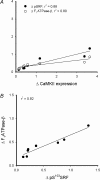
Here the hypothesis that skeletal muscle Ca(2+)-calmodulin-dependent kinase II (CaMKII) expression and signalling would be modified by endurance training was tested. Eight healthy, young men completed 3 weeks of one-legged endurance exercise training with muscle samples taken from both legs before training and 15 h after the last exercise bout. Along with an approximately 40% increase in mitochondrial F(1)-ATP synthase expression, there was an approximately 1-fold increase in maximal CaMKII activity and CaMKII kinase isoform expression after training in the active leg only. Autonomous CaMKII activity and CaMKII autophosphorylation were increased to a similar extent. However, there was no change in alpha-CaMKII anchoring protein expression with training. Nor was there any change in expression or Thr(17) phosphorylation of the CaMKII substrate phospholamban with training. However, another CaMKII substrate, serum response factor (SRF), had an approximately 60% higher phosphorylation at Ser(103) after training, with no change in SRF expression. There were positive correlations between the increases in CaMKII expression and SRF phosphorylation as well as F(1)ATPase expression with training. After training, there was an increase in cyclic-AMP response element binding protein phosphorylation at Ser(133), but not expression, in muscle of both legs. Taken together, skeletal muscle CaMKII kinase isoform expression and SRF phosphorylation is higher with endurance-type exercise training, adaptations that are restricted to active muscle. This may contribute to greater Ca(2+) mediated regulation during exercise and the altered muscle phenotype with training.
Figures
Similar articles
-
5'-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle.Am J Physiol Endocrinol Metab. 2004 Mar;286(3):E411-7. doi: 10.1152/ajpendo.00317.2003. Epub 2003 Nov 12. Am J Physiol Endocrinol Metab. 2004. PMID: 14613924 Clinical Trial.
-
Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle.J Physiol. 2003 Nov 15;553(Pt 1):303-9. doi: 10.1113/jphysiol.2003.054171. Epub 2003 Oct 17. J Physiol. 2003. PMID: 14565989 Free PMC article. Clinical Trial.
-
Divergent cell signaling after short-term intensified endurance training in human skeletal muscle.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1427-38. doi: 10.1152/ajpendo.90428.2008. Epub 2008 Sep 30. Am J Physiol Endocrinol Metab. 2008. PMID: 18827172 Clinical Trial.
-
The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis.Proc Nutr Soc. 2004 May;63(2):279-86. doi: 10.1079/PNS2004335. Proc Nutr Soc. 2004. PMID: 15294044 Review.
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
Cited by
-
The STARS signaling pathway: a key regulator of skeletal muscle function.Pflugers Arch. 2014 Sep;466(9):1659-71. doi: 10.1007/s00424-014-1475-5. Epub 2014 Feb 21. Pflugers Arch. 2014. PMID: 24557714 Review.
-
Novel pharmacological approaches to combat obesity and insulin resistance: targeting skeletal muscle with 'exercise mimetics'.Diabetologia. 2009 Oct;52(10):2015-26. doi: 10.1007/s00125-009-1420-x. Epub 2009 Jun 23. Diabetologia. 2009. PMID: 19547950 Review.
-
Training in the fasted state facilitates re-activation of eEF2 activity during recovery from endurance exercise.Eur J Appl Physiol. 2011 Jul;111(7):1297-305. doi: 10.1007/s00421-010-1753-7. Epub 2010 Dec 4. Eur J Appl Physiol. 2011. PMID: 21132439 Clinical Trial.
-
Exercise-conditioned plasma attenuates nuclear concentrations of DNA methyltransferase 3B in human peripheral blood mononuclear cells.Physiol Rep. 2015 Dec;3(12):e12621. doi: 10.14814/phy2.12621. Physiol Rep. 2015. PMID: 26660547 Free PMC article.
-
Comparison of Polarized Versus Other Types of Endurance Training Intensity Distribution on Athletes' Endurance Performance: A Systematic Review with Meta-analysis.Sports Med. 2024 Aug;54(8):2071-2095. doi: 10.1007/s40279-024-02034-z. Epub 2024 May 8. Sports Med. 2024. PMID: 38717713 Free PMC article.
References
-
- Abraham ST, Shaw C. Increased expression of δCaMKII isoforms in skeletal muscle regeneration: Implications in dystrophic muscle disease. J Cell Biochem. 2006;97:621–632. - PubMed
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. - PubMed
-
- Allen DL, Leinwand LA. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J Biol Chem. 2002;277:45323–45330. - PubMed
-
- Antipenko A, Frias JA, Parra J, Cadefau JA, Cusso R. Effect of chronic electrostimulation of rabbit skeletal muscle on calmodulin level and protein kinase activity. Int J Biochem Cell Biol. 1999;31:303–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

