Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells
- PMID: 17626215
- PMCID: PMC6672603
- DOI: 10.1523/JNEUROSCI.0431-07.2007
Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells
Abstract
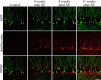
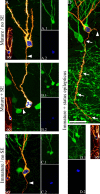
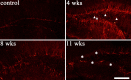
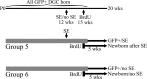
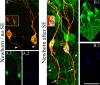
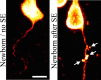
Aberrantly interconnected granule cells are characteristic of temporal lobe epilepsy. By reducing network stability, these abnormal neurons may contribute directly to disease development. Only subsets of granule cells, however, exhibit abnormalities. Why this is the case is not known. Ongoing neurogenesis in the adult hippocampus may provide an explanation. Newly generated granule cells may be uniquely vulnerable to environmental disruptions relative to their mature neighbors. Here, we determine whether there is a critical period after neuronal birth during which neuronal integration can be disrupted by an epileptogenic insult. By bromodeoxyuridine birthdating cells in green fluorescent protein-expressing transgenic mice, we were able to noninvasively label granule cells born 8 weeks before (mature), 1 week before (immature), or 3 weeks after (newborn) pilocarpine-epileptogenesis. Neuronal morphology was examined 4 and 8 weeks after pilocarpine treatment. Strikingly, almost 50% of immature granule cells exposed to pilocarpine-epileptogenesis exhibited aberrant hilar basal dendrites. In contrast, only 9% of mature granule cells exposed to the identical insult possessed basal dendrites. Moreover, newborn cells were even more severely impacted than immature cells, with 40% exhibiting basal dendrites and an additional 20% exhibiting migration defects. In comparison, <5% of neurons from normal animals exhibited either abnormality, regardless of age. Together, these data demonstrate the existence of a critical period after the birth of adult-generated neurons during which they are vulnerable to being recruited into epileptogenic neuronal circuits. Pathological brain states therefore may pose a significant hurdle for the appropriate integration of newly born endogenous, and exogenous, neurons.
Figures




Similar articles
-
Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis.Epilepsia. 2012 May;53(5):908-21. doi: 10.1111/j.1528-1167.2012.03463.x. Epilepsia. 2012. PMID: 22533643 Free PMC article.
-
Impact of rapamycin on status epilepticus induced hippocampal pathology and weight gain.Exp Neurol. 2016 Jun;280:1-12. doi: 10.1016/j.expneurol.2016.03.015. Epub 2016 Mar 17. Exp Neurol. 2016. PMID: 26995324 Free PMC article.
-
Loss of input from the mossy cells blocks maturation of newly generated granule cells.Hippocampus. 2007;17(7):510-24. doi: 10.1002/hipo.20290. Hippocampus. 2007. PMID: 17455193
-
Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy.Epilepsia. 2012 Jun;53 Suppl 1:98-108. doi: 10.1111/j.1528-1167.2012.03481.x. Epilepsia. 2012. PMID: 22612814 Review.
-
Neuroplasticity in the damaged dentate gyrus of the epileptic brain.Prog Brain Res. 2002;136:319-28. doi: 10.1016/s0079-6123(02)36027-8. Prog Brain Res. 2002. PMID: 12143392 Review.
Cited by
-
The cellular and synaptic location of activated TrkB in mouse hippocampus during limbic epileptogenesis.J Comp Neurol. 2013 Feb 15;521(3):499-521, Spc1. doi: 10.1002/cne.23225. J Comp Neurol. 2013. PMID: 22987780 Free PMC article.
-
Adult Neurogenesis in Epileptogenesis: An Update for Preclinical Finding and Potential Clinical Translation.Curr Neuropharmacol. 2020;18(6):464-484. doi: 10.2174/1570159X17666191118142314. Curr Neuropharmacol. 2020. PMID: 31744451 Free PMC article. Review.
-
Depression, stress, epilepsy and adult neurogenesis.Exp Neurol. 2012 Jan;233(1):22-32. doi: 10.1016/j.expneurol.2011.05.023. Epub 2011 Jun 12. Exp Neurol. 2012. PMID: 21684275 Free PMC article. Review.
-
Histopathological and Biochemical Assessment of Neuroprotective Effects of Sodium Valproate and Lutein on the Pilocarpine Albino Rat Model of Epilepsy.Behav Neurol. 2021 Jun 3;2021:5549638. doi: 10.1155/2021/5549638. eCollection 2021. Behav Neurol. 2021. PMID: 34149964 Free PMC article.
-
Monocyte chemoattractant protein-1 affects migration of hippocampal neural progenitors following status epilepticus in rats.J Neuroinflammation. 2013 Jan 22;10:11. doi: 10.1186/1742-2094-10-11. J Neuroinflammation. 2013. PMID: 23339567 Free PMC article.
References
-
- al-Hussain S, al-Ali S. A golgi study of cell types in the dentate gyrus of the adult human brain. Cell Mol Neurobiol. 1995;15:207–220. - PubMed
-
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. - PubMed
-
- Austin JE, Buckmaster PS. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol. 2004;476:205–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
