Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity
- PMID: 17626165
- PMCID: PMC1951766
- DOI: 10.1091/mbc.e06-07-0604
Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity
Abstract
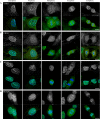
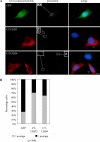
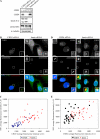
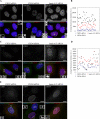
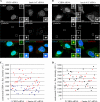
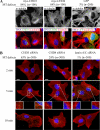
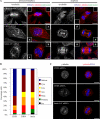
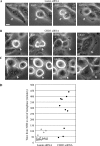
Pericentrin is an integral centrosomal component that anchors regulatory and structural molecules to centrosomes. In a yeast two-hybrid screen with pericentrin we identified chromodomain helicase DNA-binding protein 4 (CHD4/Mi2beta). CHD4 is part of the multiprotein nucleosome remodeling deacetylase (NuRD) complex. We show that many NuRD components interacted with pericentrin by coimmunoprecipitation and that they localized to centrosomes and midbodies. Overexpression of the pericentrin-binding domain of CHD4 or another family member (CHD3) dissociated pericentrin from centrosomes. Depletion of CHD3, but not CHD4, by RNA interference dissociated pericentrin and gamma-tubulin from centrosomes. Microtubule nucleation/organization, cell morphology, and nuclear centration were disrupted in CHD3-depleted cells. Spindles were disorganized, the majority showing a prometaphase-like configuration. Time-lapse imaging revealed mitotic failure before chromosome segregation and cytokinesis failure. We conclude that pericentrin forms complexes with CHD3 and CHD4, but a distinct CHD3-pericentrin complex is required for centrosomal anchoring of pericentrin/gamma-tubulin and for centrosome integrity.
Figures


Similar articles
-
Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry.Mol Biol Cell. 2004 Aug;15(8):3642-57. doi: 10.1091/mbc.e03-11-0796. Epub 2004 May 14. Mol Biol Cell. 2004. PMID: 15146056 Free PMC article.
-
CHD3 and CHD4 form distinct NuRD complexes with different yet overlapping functionality.Nucleic Acids Res. 2017 Oct 13;45(18):10534-10554. doi: 10.1093/nar/gkx711. Nucleic Acids Res. 2017. PMID: 28977666 Free PMC article.
-
A fraction of Crm1 locates at centrosomes by its CRIME domain and regulates the centrosomal localization of pericentrin.Biochem Biophys Res Commun. 2009 Jul 3;384(3):383-8. doi: 10.1016/j.bbrc.2009.04.154. Epub 2009 May 5. Biochem Biophys Res Commun. 2009. PMID: 19422798
-
Novel Mi-2 related ATP-dependent chromatin remodelers.Epigenetics. 2009 May 16;4(4):209-11. doi: 10.4161/epi.8933. Epub 2009 May 6. Epigenetics. 2009. PMID: 19535903 Review.
-
The human Mi-2/NuRD complex and gene regulation.Oncogene. 2007 Aug 13;26(37):5433-8. doi: 10.1038/sj.onc.1210611. Oncogene. 2007. PMID: 17694084 Review.
Cited by
-
The RSC chromatin-remodeling complex influences mitotic exit and adaptation to the spindle assembly checkpoint by controlling the Cdc14 phosphatase.J Cell Biol. 2010 Nov 29;191(5):981-97. doi: 10.1083/jcb.201007025. Epub 2010 Nov 22. J Cell Biol. 2010. PMID: 21098112 Free PMC article.
-
Immunological aspects of 22q11.2 deletion syndrome.Cell Mol Life Sci. 2012 Jan;69(1):17-27. doi: 10.1007/s00018-011-0842-z. Epub 2011 Oct 9. Cell Mol Life Sci. 2012. PMID: 21984609 Free PMC article. Review.
-
MBD2 and multiple domains of CHD4 are required for transcriptional repression by Mi-2/NuRD complexes.Mol Cell Biol. 2012 Dec;32(24):5078-88. doi: 10.1128/MCB.00819-12. Epub 2012 Oct 15. Mol Cell Biol. 2012. PMID: 23071088 Free PMC article.
-
MLL5 maintains genomic integrity by regulating the stability of the chromosomal passenger complex through a functional interaction with Borealin.J Cell Sci. 2012 Oct 1;125(Pt 19):4676-85. doi: 10.1242/jcs.110411. Epub 2012 Jul 13. J Cell Sci. 2012. PMID: 22797924 Free PMC article.
-
MT1-MMP regulates the PI3Kδ·Mi-2/NuRD-dependent control of macrophage immune function.Genes Dev. 2012 Feb 15;26(4):395-413. doi: 10.1101/gad.178749.111. Genes Dev. 2012. PMID: 22345520 Free PMC article.
References
-
- Araki M., Masutani C., Takemura M., Uchida A., Sugasawa K., Kondoh J., Ohkuma Y., Hanaoka F. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 2001;276:18665–18672. - PubMed
-
- Aubry F., Mattei M. G., Galibert F. Identification of a human 17p-located cDNA encoding a protein of the Snf2-like helicase family. Eur. J. Biochem. 1998;254:558–564. - PubMed
-
- Bowen N. J., Fujita N., Kajita M., Wade P. A. Mi-2/NuRD: multiple complexes for many purposes. Biochim. Biophys. Acta. 2004;1677:52–57. - PubMed
-
- Brehm A., Tufteland K. R., Aasland R., Becker P. B. The many colours of chromodomains. Bioessays. 2004;26:133–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

