Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells
- PMID: 17620408
- PMCID: PMC2064442
- DOI: 10.1083/jcb.200612127
Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells
Abstract
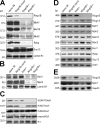
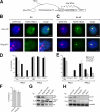
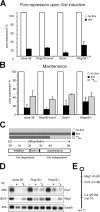
The Polycomb group (PcG) gene Ring1B has been implicated in the repression of developmental control genes and X inactivation and is essential for embryogenesis. Ring1B protein contains a RING finger domain and functions as an E3 ubiquitin ligase that is crucial for the monoubiquitination of histone H2A (H2AK119ub1). Here, we study the function of Ring1B in mouse embryonic stem (ES) cells. The deletion of Ring1B causes the loss of several PcG proteins, showing an unanticipated function in the regulation of PcG protein levels. Derepression of lineage genes and an aberrant differentiation potential is observed in Ring1B-deficient ES cells. Despite a crucial function of Ring1B in establishing the chromosome-wide ubiquitination of histone H2A lysine 119 (H2AK119ub1) upon Xist expression in ES cells, the initiation of silencing by Xist is independent of Ring1B. Other chromatin marks associated with the initiation of X inactivation are not affected in Ring1B-deficient cells, suggesting compensation for the loss of Ring1B in X inactivation in contrast to the repression of lineage genes.
Figures


Similar articles
-
Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells.PLoS One. 2008 May 21;3(5):e2235. doi: 10.1371/journal.pone.0002235. PLoS One. 2008. PMID: 18493325 Free PMC article.
-
Recruitment of PRC1 function at the initiation of X inactivation independent of PRC2 and silencing.EMBO J. 2006 Jul 12;25(13):3110-22. doi: 10.1038/sj.emboj.7601187. Epub 2006 Jun 8. EMBO J. 2006. PMID: 16763550 Free PMC article.
-
Maintenance of undifferentiated state and self-renewal of embryonic neural stem cells by Polycomb protein Ring1B.Stem Cells. 2009 Jul;27(7):1559-70. doi: 10.1002/stem.82. Stem Cells. 2009. PMID: 19544461
-
Role of polycomb proteins Ring1A and Ring1B in the epigenetic regulation of gene expression.Int J Dev Biol. 2009;53(2-3):355-70. doi: 10.1387/ijdb.082690mv. Int J Dev Biol. 2009. PMID: 19412891 Review.
-
Writing Histone Monoubiquitination in Human Malignancy-The Role of RING Finger E3 Ubiquitin Ligases.Genes (Basel). 2019 Jan 18;10(1):67. doi: 10.3390/genes10010067. Genes (Basel). 2019. PMID: 30669413 Free PMC article. Review.
Cited by
-
REST-mediated recruitment of polycomb repressor complexes in mammalian cells.PLoS Genet. 2012;8(3):e1002494. doi: 10.1371/journal.pgen.1002494. Epub 2012 Mar 1. PLoS Genet. 2012. PMID: 22396653 Free PMC article.
-
Microarray analysis of LTR retrotransposon silencing identifies Hdac1 as a regulator of retrotransposon expression in mouse embryonic stem cells.PLoS Comput Biol. 2012;8(4):e1002486. doi: 10.1371/journal.pcbi.1002486. Epub 2012 Apr 26. PLoS Comput Biol. 2012. PMID: 22570599 Free PMC article.
-
DNMT1 and DNMT3B modulate distinct polycomb-mediated histone modifications in colon cancer.Cancer Res. 2009 Sep 15;69(18):7412-21. doi: 10.1158/0008-5472.CAN-09-0116. Epub 2009 Sep 1. Cancer Res. 2009. PMID: 19723660 Free PMC article.
-
De Novo Polycomb Recruitment and Repressive Domain Formation.Epigenomes. 2022 Aug 22;6(3):25. doi: 10.3390/epigenomes6030025. Epigenomes. 2022. PMID: 35997371 Free PMC article. Review.
-
PRC1 drives Polycomb-mediated gene repression by controlling transcription initiation and burst frequency.Nat Struct Mol Biol. 2021 Oct;28(10):811-824. doi: 10.1038/s41594-021-00661-y. Epub 2021 Oct 4. Nat Struct Mol Biol. 2021. PMID: 34608337 Free PMC article.
References
-
- Arrigoni, R., S.L. Alam, J.A. Wamstad, V.J. Bardwell, W.I. Sundquist, and N. Schreiber-Agus. 2006. The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett. 580:6233–6241. - PubMed
-
- Atsuta, T., S. Fujimura, H. Moriya, M. Vidal, T. Akasaka, and H. Koseki. 2001. Production of monoclonal antibodies against mammalian Ring1B proteins. Hybridoma. 20:43–46. - PubMed
-
- Ben-Saadon, R., D. Zaaroor, T. Ziv, and A. Ciechanover. 2006. The Polycomb protein Ring1B generates self atypical mixed ubiquitin chains required for its in vitro histone H2A ligase activity. Mol. Cell. 24:701–711. - PubMed
-
- Boyer, L.A., K. Plath, J. Zeitlinger, T. Brambrink, L.A. Medeiros, T.I. Lee, S.S. Levine, M. Wernig, A. Tajonar, M.K. Ray, et al. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 441:349–353. - PubMed

