TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells
- PMID: 17617600
- PMCID: PMC2220044
- DOI: 10.4049/jimmunol.179.2.1080
TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells
Abstract
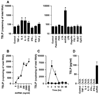
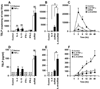
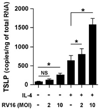
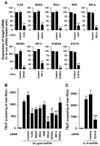
Thymic stromal lymphopoietin (TSLP) is elevated in asthma and triggers dendritic cell-mediated activation of Th2 inflammatory responses. Although TSLP has been shown to be produced mainly by airway epithelial cells, the regulation of epithelial TSLP expression has not been extensively studied. We investigated the expression of TSLP in cytokine- or TLR ligand-treated normal human bronchial epithelial cells (NHBE). The mRNA for TSLP was significantly up-regulated by stimulation with IL-4 (5.5-fold) and IL-13 (5.3-fold), weakly up-regulated by TNF-alpha, TGF-beta, and IFN-beta, and not affected by IFN-gamma in NHBE. TSLP mRNA was only significantly up-regulated by the TLR3 ligand (dsRNA) among the TLR ligands tested (66.8-fold). TSLP was also induced by in vitro infection with rhinovirus. TSLP protein was detected after stimulation with dsRNA (120 +/- 23 pg/ml). The combination of TNF-alpha and IL-4 produced detectable levels of TSLP protein (40 +/- 13 pg/ml). In addition, TSLP was synergistically enhanced by a combination of IL-4 and dsRNA (mRNA; 207-fold, protein; 325 +/- 75 pg/ml). The induction of TSLP by dsRNA was dependent upon NF-kappaB and IFN regulatory factor 3 (IRF-3) signaling via TLR3 as indicated by a study with small interfering RNA. The potent topical glucocorticoid fluticasone propionate significantly suppressed dsRNA-dependent TSLP production in NHBE. These results suggest that the expression of TSLP is induced in airway epithelial cells by stimulation with the TLR3 ligand and Th2 cytokines and that this response is suppressed by glucocorticoid treatment. This implies that respiratory viral infection and the recruitment of Th2 cytokine producing cells may amplify Th2 inflammation via the induction of TSLP in the asthmatic airway.
Figures
Similar articles
-
Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin.J Allergy Clin Immunol. 2012 Jul;130(1):225-32.e4. doi: 10.1016/j.jaci.2012.04.019. Epub 2012 May 26. J Allergy Clin Immunol. 2012. PMID: 22633328 Free PMC article.
-
Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases.J Innate Immun. 2012;4(1):86-99. doi: 10.1159/000329131. Epub 2011 Jun 20. J Innate Immun. 2012. PMID: 21691053 Clinical Trial.
-
Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma.Thorax. 2010 Jul;65(7):626-32. doi: 10.1136/thx.2009.125930. Thorax. 2010. PMID: 20627922
-
Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma.Int J Mol Sci. 2018 Apr 18;19(4):1231. doi: 10.3390/ijms19041231. Int J Mol Sci. 2018. PMID: 29670037 Free PMC article. Review.
-
Thymic stromal lymphopoietin: a promising therapeutic target for allergic diseases.Int Arch Allergy Immunol. 2013;160(1):18-26. doi: 10.1159/000341665. Epub 2012 Aug 30. Int Arch Allergy Immunol. 2013. PMID: 22948028 Review.
Cited by
-
Immunopathology of chronic rhinosinusitis.Allergol Int. 2015 Apr;64(2):121-30. doi: 10.1016/j.alit.2014.12.006. Epub 2015 Feb 9. Allergol Int. 2015. PMID: 25838086 Free PMC article. Review.
-
Selective inhibition by simvastatin of IRF3 phosphorylation and TSLP production in dsRNA-challenged bronchial epithelial cells from COPD donors.Br J Pharmacol. 2013 Jan;168(2):363-74. doi: 10.1111/j.1476-5381.2012.02131.x. Br J Pharmacol. 2013. PMID: 22881993 Free PMC article.
-
Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin.J Allergy Clin Immunol. 2016 Sep;138(3):814-824.e11. doi: 10.1016/j.jaci.2016.01.050. Epub 2016 Apr 9. J Allergy Clin Immunol. 2016. PMID: 27156176 Free PMC article.
-
The microbiology of asthma.Nat Rev Microbiol. 2012 Jun 6;10(7):459-71. doi: 10.1038/nrmicro2801. Nat Rev Microbiol. 2012. PMID: 22669219 Free PMC article. Review.
-
Thymic stromal lymphopoietin.Ann N Y Acad Sci. 2010 Jan;1183:13-24. doi: 10.1111/j.1749-6632.2009.05128.x. Ann N Y Acad Sci. 2010. PMID: 20146705 Free PMC article. Review.
References
-
- Avila PC. Interactions between allergic inflammation and respiratory viral infections. J. Allergy Clin. Immunol. 2000;106:829–831. - PubMed
-
- Johnston SL. Overview of virus-induced airway disease. Proc. Am. Thorac. Soc. 2005;2:150–156. - PubMed
-
- Contoli M, Caramori G, Mallia P, Johnston S, Papi A. Mechanisms of respiratory virus-induced asthma exacerbations. Clin. Exp. Allergy. 2005;35:137–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

