Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125)
- PMID: 17617155
- PMCID: PMC2266014
- DOI: 10.1111/j.1365-2567.2007.02660.x
Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125)
Abstract
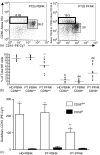
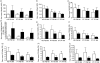
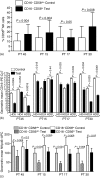
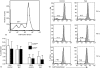
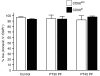
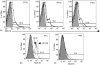
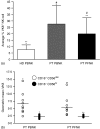
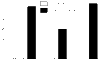
The ovarian tumour marker MUC16 (CA125) inhibits the cytotoxic responses of human natural killer (NK) cells and down-regulates CD16. Here we show that approximately 10% of the peripheral blood NK cells (PBNK) from the epithelial ovarian cancer (EOC) patients are CD16(-) CD56(br) whereas 40% of the peritoneal fluid NK (PFNK) carry this phenotype, which is usually associated with NK cells from the lymph nodes or human decidua. PBNK from healthy donors exposed to PF show a significant increase in the CD16(-) CD56(br) population. This shift in phenotype is not caused by increased apoptosis of the CD16(+) CD56(dim) cells or selective proliferation of the CD16(-) CD56(br) NK cells. Thus, the terminal differentiation of the CD16(-) CD56(br) NK cells to CD16(+) CD56(dim) subset that occurs during normal NK cell development may actually be a reversible step. A majority of the NK cell receptors (NKp46, NKp44, NKG2D, CD244, CD226, CD158a, CD158b, and CD158e) studied were down-regulated in the PFNK. MUC16 binds selectively to 30-40% of CD16(+) CD56(dim) NK cells in EOC patients indicating that phenotypic alterations in these cells are mediated by tumour-derived soluble factors. Similar to EOC, MUC16 in early pregnancy also binds to NK cells suggesting shared mechanisms of NK cell suppression in feto-maternal tolerance and immune evasion by ovarian cancers.
Figures

Similar articles
-
Terminal Differentiation of CD56(dim)CD16(+) Natural Killer Cells Is Associated with Increase in Natural Killer Cell Frequencies After Antiretroviral Treatment in HIV-1 Infection.AIDS Res Hum Retroviruses. 2015 Dec;31(12):1206-12. doi: 10.1089/aid.2015.0115. Epub 2015 Sep 9. AIDS Res Hum Retroviruses. 2015. PMID: 26352913
-
Increased frequency of ILT2-expressing CD56(dim)CD16(+) NK cells correlates with disease severity of pulmonary tuberculosis.Tuberculosis (Edinb). 2014 Sep;94(5):469-74. doi: 10.1016/j.tube.2014.03.009. Epub 2014 May 14. Tuberculosis (Edinb). 2014. PMID: 24909369
-
CD56dim CD16- Natural Killer Cell Profiling in Melanoma Patients Receiving a Cancer Vaccine and Interferon-α.Front Immunol. 2019 Jan 29;10:14. doi: 10.3389/fimmu.2019.00014. eCollection 2019. Front Immunol. 2019. PMID: 30761123 Free PMC article.
-
Extranodal NK/T cell lymphoma and aggressive NK cell leukaemia: evidence for their origin on CD56+bright CD16-/+dim NK cells.Pathology. 2015 Oct;47(6):503-14. doi: 10.1097/PAT.0000000000000275. Pathology. 2015. PMID: 26166665 Review.
-
The CD56-CD16+ NK cell subset in chronic infections.Biochem Soc Trans. 2023 Jun 28;51(3):1201-1212. doi: 10.1042/BST20221374. Biochem Soc Trans. 2023. PMID: 37140380 Review.
Cited by
-
High Density of CD16+ Tumor-Infiltrating Immune Cells in Recurrent Ovarian Cancer Is Associated with Enhanced Responsiveness to Chemotherapy and Prolonged Overall Survival.Cancers (Basel). 2021 Nov 18;13(22):5783. doi: 10.3390/cancers13225783. Cancers (Basel). 2021. PMID: 34830938 Free PMC article.
-
MUC16 as a novel target for cancer therapy.Expert Opin Ther Targets. 2018 Aug;22(8):675-686. doi: 10.1080/14728222.2018.1498845. Epub 2018 Jul 26. Expert Opin Ther Targets. 2018. PMID: 29999426 Free PMC article. Review.
-
Tumor antigen CA125 suppresses antibody-dependent cellular cytotoxicity (ADCC) via direct antibody binding and suppressed Fc-γ receptor engagement.Oncotarget. 2017 Jul 7;8(32):52045-52060. doi: 10.18632/oncotarget.19090. eCollection 2017 Aug 8. Oncotarget. 2017. PMID: 28881712 Free PMC article.
-
Expansion of CD16-negative natural killer cells in the peripheral blood of patients with metastatic melanoma.Clin Dev Immunol. 2011;2011:316314. doi: 10.1155/2011/316314. Epub 2011 Feb 23. Clin Dev Immunol. 2011. PMID: 21403861 Free PMC article.
-
Mucin1 and Mucin16: Therapeutic Targets for Cancer Therapy.Pharmaceuticals (Basel). 2021 Oct 17;14(10):1053. doi: 10.3390/ph14101053. Pharmaceuticals (Basel). 2021. PMID: 34681277 Free PMC article. Review.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Reinartz S, Wagner U. Current approaches in ovarian cancer vaccines. Minerva Ginecol. 2004;56:515–27. - PubMed
-
- Santin AD, Hermonat PL, Ravaggi A, et al. Phenotypic and functional analysis of tumor-infiltrating lymphocytes compared with tumor-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with advanced ovarian cancer. Gynecol Obstet Invest. 2001;51:254–61. - PubMed
-
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. - PubMed
-
- De Leonardis A, Casamassima A, Chiuri E, Addabbo L, De Frenza N, Falco G. [Lymphocytic subpopulations in malignant ascites of ovarian origin. Flow cytometric analysis] Minerva Ginecol. 1993;45:291–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

