Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling
- PMID: 17607356
- PMCID: PMC1891000
- DOI: 10.1172/JCI31731
Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling
Abstract
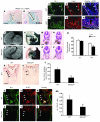
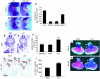
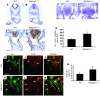
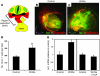
The anterior heart field (AHF), which contributes to the outflow tract and right ventricle of the heart, is defined in part by expression of the LIM homeobox transcription factor Isl-1. The importance of Isl-1-positive cells in cardiac development and homeostasis is underscored by the finding that these cells are required for cardiac development and act as cardiac stem/progenitor cells within the postnatal heart. However, the molecular pathways regulating these cells' expansion and differentiation are poorly understood. We show that Isl-1-positive AHF progenitor cells in mice were responsive to Wnt/beta-catenin signaling, and these responsive cells contributed to the outflow tract and right ventricle of the heart. Loss of Wnt/beta-catenin signaling in the AHF caused defective outflow tract and right ventricular development with a decrease in Isl-1-positive progenitors and loss of FGF signaling. Conversely, Wnt gain of function in these cells led to expansion of Isl-1-positive progenitors with a concomitant increase in FGF signaling through activation of a specific set of FGF ligands including FGF3, FGF10, FGF16, and FGF20. These data reveal what we believe to be a novel Wnt-FGF signaling axis required for expansion of Isl-1-positive AHF progenitors and suggest future therapies to increase the number and function of these cells for cardiac regeneration.
Figures


Similar articles
-
Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9313-8. doi: 10.1073/pnas.0700923104. Epub 2007 May 22. Proc Natl Acad Sci U S A. 2007. PMID: 17519333 Free PMC article.
-
Canonical Wnt signaling functions in second heart field to promote right ventricular growth.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9319-24. doi: 10.1073/pnas.0701212104. Epub 2007 May 22. Proc Natl Acad Sci U S A. 2007. PMID: 17519332 Free PMC article.
-
The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway.Cell Stem Cell. 2007 Aug 16;1(2):165-79. doi: 10.1016/j.stem.2007.05.018. Epub 2007 Jun 14. Cell Stem Cell. 2007. PMID: 18371348
-
Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades.Cancer Biol Ther. 2006 Sep;5(9):1059-64. doi: 10.4161/cbt.5.9.3151. Epub 2006 Sep 4. Cancer Biol Ther. 2006. PMID: 16940750 Review.
-
Impact of WNT signaling on tissue lineage differentiation in the early mouse embryo.Dev Growth Differ. 2011 Sep;53(7):843-56. doi: 10.1111/j.1440-169X.2011.01292.x. Epub 2011 Jul 15. Dev Growth Differ. 2011. PMID: 21762130 Review.
Cited by
-
Regenerating functional heart tissue for myocardial repair.Cell Mol Life Sci. 2012 Aug;69(16):2635-56. doi: 10.1007/s00018-012-0942-4. Epub 2012 Mar 3. Cell Mol Life Sci. 2012. PMID: 22388688 Free PMC article. Review.
-
Xin proteins and intercalated disc maturation, signaling and diseases.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2566-93. doi: 10.2741/4072. Front Biosci (Landmark Ed). 2012. PMID: 22652799 Free PMC article. Review.
-
FRS2α-mediated FGF signals suppress premature differentiation of cardiac stem cells through regulating autophagy activity.Circ Res. 2012 Feb 17;110(4):e29-39. doi: 10.1161/CIRCRESAHA.111.255950. Epub 2011 Dec 29. Circ Res. 2012. PMID: 22207710 Free PMC article.
-
Active Wnt signaling in response to cardiac injury.Basic Res Cardiol. 2010 Sep;105(5):631-41. doi: 10.1007/s00395-010-0100-9. Epub 2010 Apr 7. Basic Res Cardiol. 2010. PMID: 20373104 Free PMC article.
-
Roles of Wnt Signaling Pathway and ROR2 Receptor in Embryonic Development: An Update Review Article.Epigenet Insights. 2022 Jan 31;15:25168657211064232. doi: 10.1177/25168657211064232. eCollection 2022. Epigenet Insights. 2022. PMID: 35128307 Free PMC article.
References
-
- Kelly R.G. Molecular inroads into the anterior heart field. Trends Cardiovasc. Med. 2005;15:51–56. - PubMed
-
- Moretti A., et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. - PubMed
-
- Wu S.M., et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

