The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division
- PMID: 17606867
- PMCID: PMC2064424
- DOI: 10.1083/jcb.200611064
The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division
Abstract
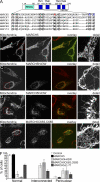
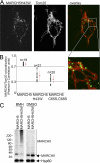
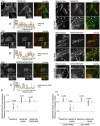
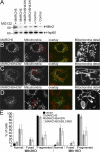
We identify a mitochondrial E3 ubiquitin ligase, MARCH5, as a critical regulator of mitochondrial fission. MARCH5 RING mutants and MARCH5 RNA interference induce an abnormal elongation and interconnection of mitochondria indicative of an inhibition of mitochondrial division. The aberrant mitochondrial phenotypes in MARCH5 RING mutant-expressing cells are reversed by ectopic expression of Drp1, but not another mitochondrial fission protein Fis1. Moreover, as indicated by abnormal clustering and mitochondrial accumulation of Drp1, as well as decreased cellular mobility of YFP-Drp1 in cells expressing MARCH5 RING mutants, MARCH5 activity regulates the subcellular trafficking of Drp1, likely by impacting the correct assembly at scission sites or the disassembly step of fission complexes. Loss of this activity may account for the observed mitochondrial division defects. Finally, MARCH5 RING mutants and endogenous Drp1, but not wild-type MARCH5 or Fis1, co-assemble into abnormally enlarged clusters in a Drp1 GTPase-dependent manner, suggesting molecular interactions among these proteins. Collectively, our data suggest a model in which mitochondrial division is regulated by a MARCH5 ubiquitin-dependent switch.
Figures





Similar articles
-
Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1.J Cell Sci. 2010 Feb 15;123(Pt 4):619-26. doi: 10.1242/jcs.061481. Epub 2010 Jan 26. J Cell Sci. 2010. PMID: 20103533 Free PMC article.
-
Novel regulatory roles of Mff and Drp1 in E3 ubiquitin ligase MARCH5-dependent degradation of MiD49 and Mcl1 and control of mitochondrial dynamics.Mol Biol Cell. 2017 Feb 1;28(3):396-410. doi: 10.1091/mbc.E16-04-0208. Epub 2016 Dec 8. Mol Biol Cell. 2017. PMID: 27932492 Free PMC article.
-
Mitochondrial E3 ubiquitin ligase MARCH5 controls mitochondrial fission and cell sensitivity to stress-induced apoptosis through regulation of MiD49 protein.Mol Biol Cell. 2016 Jan 15;27(2):349-59. doi: 10.1091/mbc.E15-09-0678. Epub 2015 Nov 12. Mol Biol Cell. 2016. PMID: 26564796 Free PMC article.
-
Roles of mitochondrial ubiquitin ligase MITOL/MARCH5 in mitochondrial dynamics and diseases.J Biochem. 2014 May;155(5):273-9. doi: 10.1093/jb/mvu016. Epub 2014 Mar 9. J Biochem. 2014. PMID: 24616159 Review.
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
Cited by
-
The Mitochondrial Ubiquitin Ligase MARCHF5 Cooperates with MCL1 to Inhibit Apoptosis in KSHV-Transformed Primary Effusion Lymphoma Cell Lines.bioRxiv [Preprint]. 2024 Sep 24:2024.09.23.614413. doi: 10.1101/2024.09.23.614413. bioRxiv. 2024. PMID: 39386614 Free PMC article. Preprint.
-
ML335 inhibits TWIK2 channel-mediated potassium efflux and attenuates mitochondrial damage in MSU crystal-induced inflammation.J Transl Med. 2024 Aug 22;22(1):785. doi: 10.1186/s12967-024-05303-7. J Transl Med. 2024. PMID: 39175013 Free PMC article.
-
Role of MARCH E3 ubiquitin ligases in cancer development.Cancer Metastasis Rev. 2024 Jul 22. doi: 10.1007/s10555-024-10201-x. Online ahead of print. Cancer Metastasis Rev. 2024. PMID: 39037545 Review.
-
MARCH5 promotes hepatocellular carcinoma progression by inducing p53 ubiquitination degradation.J Cancer Res Clin Oncol. 2024 Jun 11;150(6):303. doi: 10.1007/s00432-024-05782-7. J Cancer Res Clin Oncol. 2024. PMID: 38861187 Free PMC article.
-
3-Methyl-4-nitrophenol Exposure Deteriorates Oocyte Maturation by Inducing Spindle Instability and Mitochondrial Dysfunction.Int J Mol Sci. 2024 Mar 22;25(7):3572. doi: 10.3390/ijms25073572. Int J Mol Sci. 2024. PMID: 38612384 Free PMC article.
References
-
- Bhar, D., M.A. Karren, M. Babst, and J.M. Shaw. 2006. Dimeric Dnm1-G385D interacts with Mdv1 on mitochondria and can be stimulated to assemble into fission complexes containing Mdv1 and Fis1. J. Biol. Chem. 281:17312–17320. - PubMed
-
- Bottomley, M.J., G. Stier, D. Pennacchini, G. Legube, B. Simon, A. Akhtar, M. Sattler, and G. Musco. 2005. NMR structure of the first PHD finger of autoimmune regulator protein (AIRE1). Insights into autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) disease. J. Biol. Chem. 280:11505–11512. - PubMed
-
- Chan, D.C. 2006. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 125:1241–1252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

