Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes
- PMID: 17604100
- PMCID: PMC2039774
- DOI: 10.1016/j.biomaterials.2007.06.001
Interactive effects of surface topography and pulsatile electrical field stimulation on orientation and elongation of fibroblasts and cardiomyocytes
Abstract
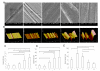
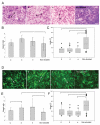
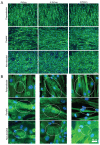
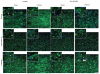
In contractile tissues such as myocardium, functional properties are directly related to the cellular orientation and elongation. Thus, tissue engineering of functional cardiac patches critically depends on our understanding of the interaction between multiple guidance cues such as topographical, adhesive or electrical. The main objective of this study was to determine the interactive effects of contact guidance and electrical field stimulation on elongation and orientation of fibroblasts and cardiomyocytes, major cell populations of the myocardium. Polyvinyl surfaces were abraded using lapping paper with grain size 1-80 microm, resulting in V-shaped abrasions with the average abrasion peak-to-peak width in the range from 3 to 13 microm, and the average depth in the range from 140 to 700 nm (AFM). The surfaces with abrasions 13 microm wide and 700 nm deep, exhibited the strongest effect on neonatal rat cardiomyocyte elongation and orientation as well as statistically significant effect on orientation of fibroblasts, thus they were utilized for electrical field stimulation. Electrical field stimulation was performed using a regime of relevance for heart tissue in vivo as well as for cardiac tissue engineering. Stimulation (square pulses, 1 ms duration, 1 Hz, 2.3 or 4.6 V/cm) was initiated 24 h after cell seeding and maintained for additional 72 h. The cover slips were positioned between the carbon rod electrodes such that the abrasions were either parallel or perpendicular to the field lines. Non-abraded surfaces were utilized as controls. Field stimulation did not affect cell viability. The presence of a well-developed contractile apparatus in neonatal rat cardiomyocytes (staining for cardiac Troponin I and actin filaments) was identified in the groups cultivated on abraded surfaces in the presence of field stimulation. Overall we observed that (i) fibroblast and cardiomyocyte elongation on non-abraded surfaces was significantly enhanced by electrical field stimulation, (ii) electrical field stimulation promoted orientation of fibroblasts in the direction perpendicular to the field lines when the abrasions were also placed perpendicular to the field lines and (iii) topographical cues were a significantly stronger determinant of cardiomyocyte orientation than the electrical field stimulation. The orientation and elongation response of cardiomyocytes was completely abolished by inhibition of actin polymerization (Cytochalasin D) and only partially by inhibition of phosphatidyl-inositol 3 kinase (PI3K) pathway (LY294002).
Figures




Similar articles
-
Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes.Lab Chip. 2009 Feb 21;9(4):564-75. doi: 10.1039/b810034a. Epub 2008 Nov 19. Lab Chip. 2009. PMID: 19190792
-
Electric field stimulation integrated into perfusion bioreactor for cardiac tissue engineering.Tissue Eng Part C Methods. 2010 Dec;16(6):1417-26. doi: 10.1089/ten.TEC.2010.0068. Epub 2010 May 10. Tissue Eng Part C Methods. 2010. PMID: 20367291
-
Engineering of oriented myocardium on three-dimensional micropatterned collagen-chitosan hydrogel.Int J Artif Organs. 2012 Apr 30;35(4):237-50. doi: 10.5301/ijao.5000084. Epub 2012 Apr 13. Int J Artif Organs. 2012. PMID: 22505198
-
Electrical stimulation through conductive scaffolds for cardiomyocyte tissue engineering: Systematic review and narrative synthesis.Ann N Y Acad Sci. 2022 Sep;1515(1):105-119. doi: 10.1111/nyas.14812. Epub 2022 Jun 8. Ann N Y Acad Sci. 2022. PMID: 35676231 Free PMC article. Review.
-
Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling.Pflugers Arch. 2011 Jul;462(1):105-17. doi: 10.1007/s00424-011-0931-8. Epub 2011 Feb 11. Pflugers Arch. 2011. PMID: 21308471 Free PMC article. Review.
Cited by
-
PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues.Biomaterials. 2013 Sep;34(27):6355-66. doi: 10.1016/j.biomaterials.2013.04.045. Epub 2013 Jun 6. Biomaterials. 2013. PMID: 23747008 Free PMC article.
-
PI3K Phosphorylation Is Linked to Improved Electrical Excitability in an In Vitro Engineered Heart Tissue Disease Model System.Tissue Eng Part A. 2015 Sep;21(17-18):2379-89. doi: 10.1089/ten.TEA.2014.0412. Epub 2015 Aug 7. Tissue Eng Part A. 2015. PMID: 26120935 Free PMC article.
-
The integrative aspects of cardiac physiology and their implications for cell-based therapy.Ann N Y Acad Sci. 2010 Feb;1188:7-14. doi: 10.1111/j.1749-6632.2009.05077.x. Ann N Y Acad Sci. 2010. PMID: 20201880 Free PMC article.
-
Bioengineering heart muscle: a paradigm for regenerative medicine.Annu Rev Biomed Eng. 2011 Aug 15;13:245-67. doi: 10.1146/annurev-bioeng-071910-124701. Annu Rev Biomed Eng. 2011. PMID: 21568715 Free PMC article.
-
Electrical stimulation as a biomimicry tool for regulating muscle cell behavior.Organogenesis. 2013 Apr-Jun;9(2):87-92. doi: 10.4161/org.25121. Epub 2013 Apr 1. Organogenesis. 2013. PMID: 23823664 Free PMC article. Review.
References
-
- Berger HJ, Prasad SK, Davidoff AJ, Pimental D, Ellingsen O, Marsh JD, et al. Continual electric field stimulation preserves contractile function of adult ventricular myocytes in primary culture. Am J Physiol. 1994;266:H341–9. - PubMed
-
- Holt E, Lunde PK, Sejersted OM, Christensen G. Electrical stimulation of adult rat cardiomyocytes in culture improves contractile properties and is associated with altered calcium handling. Basic Res Cardiol. 1997;92:289–98. - PubMed
-
- Johnson TB, Kent RL, Bubolz BA, McDermott PJ. Electrical stimulation of contractile activity accelerates growth of cultured neonatal cardiocytes. Circ Res. 1994;74:448–59. - PubMed
-
- Ivester CT, Tuxworth WJ, Cooper Gt, McDermott PJ. Contraction accelerates myosin heavy chain synthesis rates in adult cardiocytes by an increase in the rate of translational initiation. J Biol Chem. 1995;270:21950–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

