The Caenorhabditis elegans septin complex is nonpolar
- PMID: 17599066
- PMCID: PMC1933406
- DOI: 10.1038/sj.emboj.7601775
The Caenorhabditis elegans septin complex is nonpolar
Abstract
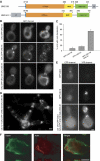
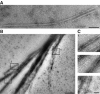
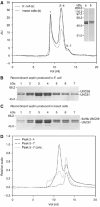
Septins are conserved GTPases that form heteromultimeric complexes and assemble into filaments that play a critical role in cell division and polarity. Results from budding and fission yeast indicate that septin complexes form around a tetrameric core. However, the molecular structure of the core and its influence on the polarity of septin complexes and filaments is poorly defined. The septin complex of the nematode Caenorhabditis elegans is formed entirely by the core septins UNC-59 and UNC-61. We show that UNC-59 and UNC-61 form a dimer of coiled-coil-mediated heterodimers. By electron microscopy, this heterotetramer appears as a linear arrangement of four densities representing the four septin subunits. Fusion of GFP to the N termini of UNC-59 and UNC-61 and subsequent electron microscopic visualization suggests that the sequence of septin subunits is UNC-59/UNC-61/UNC-61/UNC-59. Visualization of GFP extensions fused to the extremity of the C-terminal coiled coils indicates that these extend laterally from the heterotetrameric core. Together, our study establishes that the septin core complex is symmetric, and suggests that septins form nonpolar filaments.
Figures



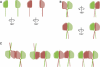
Similar articles
-
Structural insight into filament formation by mammalian septins.Nature. 2007 Sep 20;449(7160):311-5. doi: 10.1038/nature06052. Epub 2007 Jul 18. Nature. 2007. PMID: 17637674
-
The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis.J Cell Sci. 2000 Nov;113 Pt 21:3825-37. doi: 10.1242/jcs.113.21.3825. J Cell Sci. 2000. PMID: 11034910
-
Human septin-septin interactions as a prerequisite for targeting septin complexes in the cytosol.Biochem J. 2004 Sep 15;382(Pt 3):783-91. doi: 10.1042/BJ20040372. Biochem J. 2004. PMID: 15214843 Free PMC article.
-
Some assembly required: yeast septins provide the instruction manual.Trends Cell Biol. 2005 Aug;15(8):414-24. doi: 10.1016/j.tcb.2005.06.007. Trends Cell Biol. 2005. PMID: 16009555 Free PMC article. Review.
-
Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast.Cell Cycle. 2009 Jan 15;8(2):195-203. doi: 10.4161/cc.8.2.7381. Cell Cycle. 2009. PMID: 19164941 Free PMC article. Review.
Cited by
-
Seeking truth on Monte Verita. Workshop on the molecular biology and biochemistry of septins and septin function.EMBO Rep. 2007 Dec;8(12):1120-6. doi: 10.1038/sj.embor.7401116. Epub 2007 Nov 2. EMBO Rep. 2007. PMID: 17975554 Free PMC article. No abstract available.
-
Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules.J Cell Biol. 2013 Dec 23;203(6):895-905. doi: 10.1083/jcb.201308068. J Cell Biol. 2013. PMID: 24344182 Free PMC article.
-
Septin Assembly and Remodeling at the Cell Division Site During the Cell Cycle.Front Cell Dev Biol. 2021 Nov 25;9:793920. doi: 10.3389/fcell.2021.793920. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901034 Free PMC article. Review.
-
Septin assemblies form by diffusion-driven annealing on membranes.Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):2146-51. doi: 10.1073/pnas.1314138111. Epub 2014 Jan 27. Proc Natl Acad Sci U S A. 2014. PMID: 24469790 Free PMC article.
-
The evolution, complex structures and function of septin proteins.Cell Mol Life Sci. 2009 Oct;66(20):3309-23. doi: 10.1007/s00018-009-0087-2. Epub 2009 Jul 14. Cell Mol Life Sci. 2009. PMID: 19597764 Free PMC article. Review.
References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smth JA, Struhl K (eds). (1987) Current Protocols in Molecular Biology. Harvard Medical School, Boston: John Wiley and Sons
-
- Barral Y, Mermall V, Mooseker MS, Snyder M (2000) Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell 5: 841–851 - PubMed
-
- Beites CL, Xie H, Bowser R, Trimble WS (1999) The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci 2: 434–439 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

