SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway
- PMID: 17591960
- PMCID: PMC1941583
- DOI: 10.2353/ajpath.2007.051264
SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway
Abstract
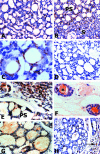
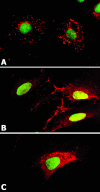
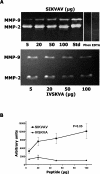
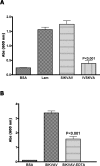
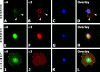
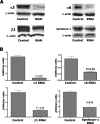
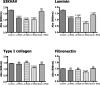
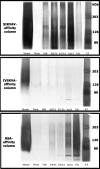
Adenoid cystic carcinoma is a frequently occurring malignant salivary gland neoplasm. We studied the induction of protease activity by the laminin-derived peptide, SIKVAV, in cells (CAC2) derived from this neoplasm. Laminin alpha1 and matrix metalloproteinases (MMPs) 2 and 9 were immunolocalized in adenoid cystic carcinoma cells in vivo and in vitro. CAC2 cells cultured on SIKVAV showed a dose-dependent increase of MMP9 as detected by zymography and colocalization of alpha3 and alpha6 integrins. Small interfering RNA (siRNA) knockdown of integrin expression in CAC2 cells resulted in decreased adhesion to the peptide. SIKVAV affinity chromatography and immunoblot analysis showed that alpha3, alpha6, and beta1 integrins were eluted from the SIKVAV column, which was confirmed by mass spectrometry and a solid-phase binding assay. Small interfering RNA experiments also showed that these integrins, through extracellular signal-regulated kinase (ERK) 1/2 signaling, regulate MMP secretion induced by SIKVAV in CAC2 cells. We propose that SIKVAV increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through alpha3beta1 and alpha6beta1 integrins and the ERK 1/2 signaling pathway.
Figures

Similar articles
-
Adhesion and protease activity in cell lines from human salivary gland tumors are regulated by the laminin-derived peptide AG73, syndecan-1 and beta1 integrin.Matrix Biol. 2008 Jun;27(5):402-19. doi: 10.1016/j.matbio.2008.02.007. Epub 2008 Feb 29. Matrix Biol. 2008. PMID: 18378436
-
Laminin-1 and SIKVAV a laminin-1-derived peptide, regulate the morphology and protease activity of a human salivary gland adenoid cystic carcinoma cell line.Oral Oncol. 2004 May;40(5):483-9. doi: 10.1016/j.oraloncology.2003.10.002. Oral Oncol. 2004. PMID: 15006619
-
The effect of laminin and its peptide SIKVAV on a human salivary gland adenoid cystic carcinoma cell line.Virchows Arch. 2002 Dec;441(6):569-76. doi: 10.1007/s00428-002-0678-x. Epub 2002 Aug 9. Virchows Arch. 2002. PMID: 12461614
-
Malignancy-related 67kDa laminin receptor in adenoid cystic carcinoma. Effect on migration and beta-catenin expression.Oral Oncol. 2007 Nov;43(10):987-98. doi: 10.1016/j.oraloncology.2006.11.005. Epub 2007 Jan 25. Oral Oncol. 2007. PMID: 17257887
-
Basement membrane and the SIKVAV laminin-derived peptide promote tumor growth and metastases.Cancer Metastasis Rev. 1991 Oct;10(3):245-54. doi: 10.1007/BF00050795. Cancer Metastasis Rev. 1991. PMID: 1764767 Review.
Cited by
-
Artificial extracellular matrix scaffolds of mobile molecules enhance maturation of human stem cell-derived neurons.Cell Stem Cell. 2023 Feb 2;30(2):219-238.e14. doi: 10.1016/j.stem.2022.12.010. Epub 2023 Jan 12. Cell Stem Cell. 2023. PMID: 36638801 Free PMC article.
-
Fragments of extracellular matrix as mediators of inflammation.Int J Biochem Cell Biol. 2008;40(6-7):1101-10. doi: 10.1016/j.biocel.2007.12.005. Epub 2007 Dec 24. Int J Biochem Cell Biol. 2008. PMID: 18243041 Free PMC article. Review.
-
Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure.Assay Drug Dev Technol. 2013 Mar;11(2):70-92. doi: 10.1089/adt.2012.474. Epub 2012 Oct 9. Assay Drug Dev Technol. 2013. PMID: 23046407 Free PMC article. Review.
-
Expression of integrin alpha6beta1 enhances tumorigenesis in glioma cells.Am J Pathol. 2009 Aug;175(2):844-55. doi: 10.2353/ajpath.2009.080920. Epub 2009 Jul 2. Am J Pathol. 2009. PMID: 19574430 Free PMC article.
-
Enteric Species F Human Adenoviruses use Laminin-Binding Integrins as Co-Receptors for Infection of Ht-29 Cells.Sci Rep. 2018 Jul 3;8(1):10019. doi: 10.1038/s41598-018-28255-7. Sci Rep. 2018. PMID: 29968781 Free PMC article.
References
-
- Seifert G, Sobin LH. The World Health Organization’s Histological Classification of Salivary Gland Tumors: a commentary on the second edition. Cancer. 1992;70:379–385. - PubMed
-
- Dardick I. Salivary Gland Tumor Pathology. New York: Igaku-Shoin,; 1996
-
- Loducca SV, Raitz R, Araujo NS, Araujo VC. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: distinct architectural composition revealed by collagen IV, laminin and their integrin ligands (α2β1 and α3β1). Histopathology. 2000;37:118–123. - PubMed
-
- Cheng J, Irie T, Munakata R, Kimura S, Nakamura H, He RG, Lui AR, Saku T. Biosynthesis of basement membrane molecules by salivary adenoid cystic carcinoma cells: an immunofluorescence and confocal microscopic study. Virchows Arch. 1995;426:577–586. - PubMed
-
- Cheng J, Saku T, Okabe H, Furthmayr H. Basement membranes in adenoid cystic carcinoma. An immunohistochemical study. Cancer. 1992;69:2631–2640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

