Altered ATP7A expression and other compensatory responses in a murine model of Menkes disease
- PMID: 17588765
- PMCID: PMC2040029
- DOI: 10.1016/j.nbd.2007.05.004
Altered ATP7A expression and other compensatory responses in a murine model of Menkes disease
Abstract
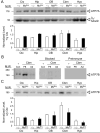
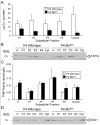
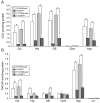
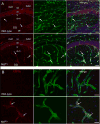
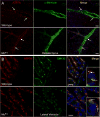
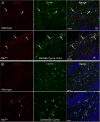
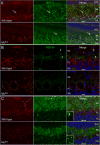
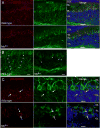
Mutations in the copper-transporter ATP7A lead to severe neurodegeneration in the mottled brindled hemizygous male (MoBr/y) mouse and human patients with Menkes disease. Our earlier studies demonstrated cell-type- and -stage-specific changes in ATP7A protein expression during postnatal neurodevelopment. Here we examined copper and cuproenzyme levels in MoBr/y mice to search for compensatory responses. While all MoBr/y neocortical subcellular fractions had decreased copper levels, the greatest decrease (8-fold) was observed in cytosol. Immunostaining for ATP7A revealed decreased levels in MoBr/y hippocampal pyramidal and cerebellar Purkinje neurons. In contrast, an upregulation of ATP7A protein occurred in MoBr/y endothelial cells, perhaps to compensate for a lack of copper in the neuropil. MoBr/y astrocytes and microglia increased their physical association with the blood-brain barrier. No alterations in ATP7A levels were observed in ependymal cells, arguing for specificity in the alteration observed at the blood-brain barrier. The decreased expression of ATP7A protein in MoBr/y Purkinje cells was associated with impaired synaptogenesis and dramatic cytoskeletal dysfunction. Immunoblotting failed to reveal any compensatory increase in levels of ATP7B. While total levels of several cuproenzymes (peptide-amidating monooxygenase, SOD1, and SOD3) were unaltered in the MoBr/y brain, levels of amidated cholecystokinin (CCK8) and amidated pituitary adenylate cyclase-activating polypeptide (PACAP38) were reduced in a tissue-specific fashion. The compensatory changes observed in the neurovascular unit provide insight into the success of copper injections within a defined neurodevelopmental period.
Figures
Similar articles
-
Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment.Neuroscience. 2006;139(3):947-64. doi: 10.1016/j.neuroscience.2006.01.044. Epub 2006 Mar 23. Neuroscience. 2006. PMID: 16549268
-
Small amounts of functional ATP7A protein permit mild phenotype.J Trace Elem Med Biol. 2015;31:173-7. doi: 10.1016/j.jtemb.2014.07.022. Epub 2014 Aug 8. J Trace Elem Med Biol. 2015. PMID: 25172213 Review.
-
Mutation in the CPC motif-containing 6th transmembrane domain affects intracellular localization, trafficking and copper transport efficiency of ATP7A protein in mosaic mutant mice--an animal model of Menkes disease.Metallomics. 2012 Feb;4(2):197-204. doi: 10.1039/c1mt00134e. Epub 2011 Nov 16. Metallomics. 2012. PMID: 22089129
-
Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein, in cells from blotchy (Mo blo) and brindled (Mo br) mouse mutants.Hum Mol Genet. 1999 Jun;8(6):1069-75. doi: 10.1093/hmg/8.6.1069. Hum Mol Genet. 1999. PMID: 10332039
-
ATP7A-related copper transport diseases-emerging concepts and future trends.Nat Rev Neurol. 2011 Jan;7(1):15-29. doi: 10.1038/nrneurol.2010.180. Nat Rev Neurol. 2011. PMID: 21221114 Free PMC article. Review.
Cited by
-
Copper dependent ERK1/2 phosphorylation is essential for the viability of neurons and not glia.Metallomics. 2022 Apr 1;14(4):mfac005. doi: 10.1093/mtomcs/mfac005. Metallomics. 2022. PMID: 35150272 Free PMC article.
-
Systemic Copper Disorders Influence the Olfactory Function in Adult Rats: Roles of Altered Adult Neurogenesis and Neurochemical Imbalance.Biomolecules. 2021 Sep 6;11(9):1315. doi: 10.3390/biom11091315. Biomolecules. 2021. PMID: 34572528 Free PMC article.
-
The Role of Copper Homeostasis in Brain Disease.Int J Mol Sci. 2022 Nov 10;23(22):13850. doi: 10.3390/ijms232213850. Int J Mol Sci. 2022. PMID: 36430330 Free PMC article. Review.
-
Peptidylglycine α-amidating monooxygenase heterozygosity alters brain copper handling with region specificity.J Neurochem. 2013 Dec;127(5):605-19. doi: 10.1111/jnc.12438. Epub 2013 Oct 13. J Neurochem. 2013. PMID: 24032518 Free PMC article.
-
Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism.Nutrients. 2019 Jun 17;11(6):1364. doi: 10.3390/nu11061364. Nutrients. 2019. PMID: 31213024 Free PMC article. Review.
References
-
- Barnes N, Tsivkovskii R, Tsivkovskaia N, Lutsenko S. The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem. 2005;280:9640–9645. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. - PMC - PubMed
-
- Bertrand E, Lewandowska E, Szpak GM, Hoogenraad T, Blaauwgers HG, Czlonkowska A, Dymecki J. Neuropathological analysis of pathological forms of astroglia in Wilson’s disease. Folia Neuropathol. 2001;39:73–79. - PubMed
-
- Brown DR. Metallic prions. Biochem Soc Symp. 2004:193–202. - PubMed
-
- Bush AI. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003;26:207–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

