Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway
- PMID: 17582000
- PMCID: PMC1951437
- DOI: 10.1128/JVI.00537-07
Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway
Abstract
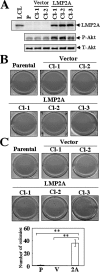
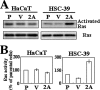
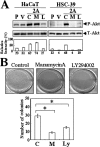
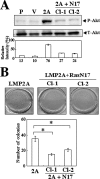
Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) is widely expressed in EBV-infected cells within the infected human host and EBV-associated malignancies, suggesting that LMP2A is important for EBV latency, persistence, and EBV-associated tumorigenesis. Previously, we demonstrated that LMP2A provides an antiapoptotic signal through the activation of phosphatidylinositol 3-kinase (PI3-K)/Akt pathway in vitro. However, the exact function of LMP2A in tumor progression is not well understood. In this study, we found that LMP2A did not induce anchorage-independent cell growth in a human keratinocyte cell line, HaCaT, but did in a human gastric carcinoma cell line, HSC-39. In addition, LMP2A activated the PI3-K/Akt pathway in both HaCaT and HSC-39 cells; however, LMP2A did not activate Ras in HaCaT cells but did in HSC-39 cells. Furthermore, the Ras inhibitors manumycin A and a dominant-negative form of Ras (RasN17) and the PI3-K inhibitor LY294002 blocked LMP2A-mediated Akt phosphorylation and anchorage-independent cell growth in HSC-39 cells. These results suggest that constitutive activation of the Ras/PI3-K/Akt pathway by LMP2A is a key factor for LMP2A-mediated transformation.
Figures

Similar articles
-
Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway.Oncogene. 2004 Nov 11;23(53):8619-28. doi: 10.1038/sj.onc.1207905. Oncogene. 2004. PMID: 15361852
-
Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt.J Virol. 2000 Nov;74(22):10681-9. doi: 10.1128/jvi.74.22.10681-10689.2000. J Virol. 2000. PMID: 11044112 Free PMC article.
-
Epstein-Barr virus upregulates phosphorylated heat shock protein 27 kDa in carcinoma cells using the phosphoinositide 3-kinase/Akt pathway.Electrophoresis. 2008 Aug;29(15):3192-200. doi: 10.1002/elps.200800086. Electrophoresis. 2008. PMID: 18604870
-
The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer.Cell Mol Biol Lett. 2009;14(2):222-47. doi: 10.2478/s11658-008-0045-2. Epub 2008 Dec 13. Cell Mol Biol Lett. 2009. PMID: 19082921 Free PMC article. Review.
-
Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction.Infect Agents Dis. 1994 Apr-Jun;3(2-3):128-36. Infect Agents Dis. 1994. PMID: 7812651 Review.
Cited by
-
EBV-associated diseases: Current therapeutics and emerging technologies.Front Immunol. 2022 Oct 27;13:1059133. doi: 10.3389/fimmu.2022.1059133. eCollection 2022. Front Immunol. 2022. PMID: 36389670 Free PMC article. Review.
-
Epstein-Barr virus latent membrane protein-2A induces ITAM/Syk- and Akt-dependent epithelial migration through αv-integrin membrane translocation.J Virol. 2012 Oct;86(19):10308-20. doi: 10.1128/JVI.00853-12. Epub 2012 Jul 25. J Virol. 2012. PMID: 22837212 Free PMC article.
-
Epstein-Barr virus latent membrane protein-2A-induced DeltaNp63alpha expression is associated with impaired epithelial-cell differentiation.Oncogene. 2010 Jul 29;29(30):4287-96. doi: 10.1038/onc.2010.175. Epub 2010 May 24. Oncogene. 2010. PMID: 20498633 Free PMC article.
-
Transcriptome sequencing of LMP2A-transfected gastric cancer cells identifies potential biomarkers in EBV-associated gastric cancer.Virus Genes. 2022 Dec;58(6):515-526. doi: 10.1007/s11262-022-01925-5. Epub 2022 Jul 11. Virus Genes. 2022. PMID: 35819701
-
Role of the immunoreceptor tyrosine-based activation motif of latent membrane protein 2A (LMP2A) in Epstein-Barr virus LMP2A-induced cell transformation.J Virol. 2014 May;88(9):5189-94. doi: 10.1128/JVI.03714-13. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554661 Free PMC article.
References
-
- Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. - PubMed
-
- Bell, A. I., K. Groves, G. L. Kelly, D. Croom-Carter, E. Hui, A. T. Chan, and A. B. Rickinson. 2006. Analysis of Epstein-Barr virus latent gene expression in endemic Burkitt's lymphoma and nasopharyngeal carcinoma tumour cells by using quantitative real-time PCR assays. J. Gen. Virol. 87:2885-2890. - PubMed
-
- Boukamp, P., E. J. Stanbridge, D. Y. Foo, P. A. Cerutti, and N. E. Fusenig. 1990. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 50:2840-2847. - PubMed
-
- Breitkreutz, D., P. Boukamp, C. M. Ryle, H. J. Stark, D. R. Roop, and N. E. Fusenig. 1991. Epidermal morphogenesis and keratin expression in c-Ha-ras-transfected tumorigenic clones of the human HaCaT cell line. Cancer Res. 51:4402-4409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

