PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1
- PMID: 17579517
- PMCID: PMC1892574
- DOI: 10.1371/journal.pbio.0050172
PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1
Abstract
Mutations in the PTEN induced putative kinase 1 (PINK1) gene cause an autosomal recessive form of Parkinson disease (PD). So far, no substrates of PINK1 have been reported, and the mechanism by which PINK1 mutations lead to neurodegeneration is unknown. Here we report the identification of TNF receptor-associated protein 1 (TRAP1), a mitochondrial molecular chaperone also known as heat shock protein 75 (Hsp75), as a cellular substrate for PINK1 kinase. PINK1 binds and colocalizes with TRAP1 in the mitochondria and phosphorylates TRAP1 both in vitro and in vivo. We show that PINK1 protects against oxidative-stress-induced cell death by suppressing cytochrome c release from mitochondria, and this protective action of PINK1 depends on its kinase activity to phosphorylate TRAP1. Moreover, we find that the ability of PINK1 to promote TRAP1 phosphorylation and cell survival is impaired by PD-linked PINK1 G309D, L347P, and W437X mutations. Our findings suggest a novel pathway by which PINK1 phosphorylates downstream effector TRAP1 to prevent oxidative-stress-induced apoptosis and implicate the dysregulation of this mitochondrial pathway in PD pathogenesis.
Conflict of interest statement
Figures
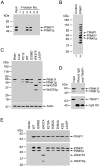

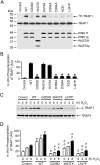
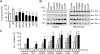
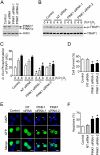
Comment in
-
Mitochondrial cell death control in familial Parkinson disease.PLoS Biol. 2007 Jul;5(7):e206. doi: 10.1371/journal.pbio.0050206. Epub 2007 Jul 17. PLoS Biol. 2007. PMID: 17638420 Free PMC article.
Similar articles
-
TRAP1 rescues PINK1 loss-of-function phenotypes.Hum Mol Genet. 2013 Jul 15;22(14):2829-41. doi: 10.1093/hmg/ddt132. Epub 2013 Mar 21. Hum Mol Genet. 2013. PMID: 23525905 Free PMC article.
-
Impairment of oxidative stress-induced heme oxygenase-1 expression by the defect of Parkinson-related gene of PINK1.J Neurochem. 2011 May;117(4):643-53. doi: 10.1111/j.1471-4159.2011.07229.x. Epub 2011 Apr 1. J Neurochem. 2011. PMID: 21366594
-
Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1.Hum Mol Genet. 2008 Feb 15;17(4):602-16. doi: 10.1093/hmg/ddm334. Epub 2007 Nov 14. Hum Mol Genet. 2008. PMID: 18003639
-
Analysis of the regulatory and catalytic domains of PTEN-induced kinase-1 (PINK1).Hum Mutat. 2012 Oct;33(10):1408-22. doi: 10.1002/humu.22127. Epub 2012 Jul 5. Hum Mutat. 2012. PMID: 22644621 Review.
-
PINK1 signaling in mitochondrial homeostasis and in aging (Review).Int J Mol Med. 2017 Jan;39(1):3-8. doi: 10.3892/ijmm.2016.2827. Epub 2016 Dec 12. Int J Mol Med. 2017. PMID: 27959386 Review.
Cited by
-
PINK1 protects against cell death induced by mitochondrial depolarization, by phosphorylating Bcl-xL and impairing its pro-apoptotic cleavage.Cell Death Differ. 2013 Jul;20(7):920-30. doi: 10.1038/cdd.2013.19. Epub 2013 Mar 22. Cell Death Differ. 2013. PMID: 23519076 Free PMC article.
-
Inactivation of Pink1 gene in vivo sensitizes dopamine-producing neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and can be rescued by autosomal recessive Parkinson disease genes, Parkin or DJ-1.J Biol Chem. 2012 Jun 29;287(27):23162-70. doi: 10.1074/jbc.M112.346437. Epub 2012 Apr 17. J Biol Chem. 2012. PMID: 22511790 Free PMC article.
-
Activation of mitochondrial TRAP1 stimulates mitochondria-lysosome crosstalk and correction of lysosomal dysfunction.iScience. 2022 Aug 14;25(9):104941. doi: 10.1016/j.isci.2022.104941. eCollection 2022 Sep 16. iScience. 2022. PMID: 36065186 Free PMC article.
-
Mitophagy in the aging nervous system.Front Cell Dev Biol. 2022 Oct 11;10:978142. doi: 10.3389/fcell.2022.978142. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36303604 Free PMC article. Review.
-
Hsp90 regulation of mitochondrial protein folding: from organelle integrity to cellular homeostasis.Cell Mol Life Sci. 2013 Jul;70(14):2463-72. doi: 10.1007/s00018-012-1177-0. Epub 2012 Sep 30. Cell Mol Life Sci. 2013. PMID: 23052217 Free PMC article.
References
-
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. - PubMed
-
- Farrer MJ. Genetics of Parkinson disease: Paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. - PubMed
-
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. - PubMed
-
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24:308–318. - PubMed
-
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

