BACE1 regulates voltage-gated sodium channels and neuronal activity
- PMID: 17576410
- PMCID: PMC2747787
- DOI: 10.1038/ncb1602
BACE1 regulates voltage-gated sodium channels and neuronal activity
Abstract
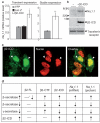
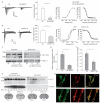
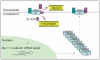
BACE1 activity is significantly increased in the brains of Alzheimer's disease patients, potentially contributing to neurodegeneration. The voltage-gated sodium channel (Na(v)1) beta2-subunit (beta2), a type I membrane protein that covalently binds to Na(v)1 alpha-subunits, is a substrate for BACE1 and gamma-secretase. Here, we find that BACE1-gamma-secretase cleavages release the intracellular domain of beta2, which increases mRNA and protein levels of the pore-forming Na(v)1.1 alpha-subunit in neuroblastoma cells. Similarly, endogenous beta2 processing and Na(v)1.1 protein levels are elevated in brains of BACE1-transgenic mice and Alzheimer's disease patients with high BACE1 levels. However, Na(v)1.1 is retained inside the cells and cell surface expression of the Na(v)1 alpha-subunits and sodium current densities are markedly reduced in both neuroblastoma cells and adult hippocampal neurons from BACE1-transgenic mice. BACE1, by cleaving beta2, thus regulates Na(v)1 alpha-subunit levels and controls cell-surface sodium current densities. BACE1 inhibitors may normalize membrane excitability in Alzheimer's disease patients with elevated BACE1 activity.
Figures


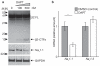

Similar articles
-
Reduced sodium channel Na(v)1.1 levels in BACE1-null mice.J Biol Chem. 2011 Mar 11;286(10):8106-8116. doi: 10.1074/jbc.M110.134692. Epub 2010 Dec 29. J Biol Chem. 2011. PMID: 21190943 Free PMC article.
-
Alzheimer's secretases regulate voltage-gated sodium channels.Neurosci Lett. 2010 Dec 10;486(2):68-72. doi: 10.1016/j.neulet.2010.08.048. Epub 2010 Sep 15. Neurosci Lett. 2010. PMID: 20817076 Free PMC article. Review.
-
Non-proteolytic effect of beta-site APP-cleaving enzyme 1 (BACE1) on sodium channel function.Neurobiol Dis. 2009 Feb;33(2):282-9. doi: 10.1016/j.nbd.2008.10.015. Epub 2008 Nov 8. Neurobiol Dis. 2009. PMID: 19056495
-
β-Site APP-cleaving enzyme 1 (BACE1) cleaves cerebellar Na+ channel β4-subunit and promotes Purkinje cell firing by slowing the decay of resurgent Na+ current.Pflugers Arch. 2011 Mar;461(3):355-71. doi: 10.1007/s00424-010-0913-2. Epub 2011 Jan 19. Pflugers Arch. 2011. PMID: 21246381
-
Ion channel regulation by β-secretase BACE1 - enzymatic and non-enzymatic effects beyond Alzheimer's disease.Channels (Austin). 2016 Sep 2;10(5):365-378. doi: 10.1080/19336950.2016.1196307. Epub 2016 Jun 2. Channels (Austin). 2016. PMID: 27253079 Free PMC article. Review.
Cited by
-
Inhibiting BACE1 to reverse synaptic dysfunctions in Alzheimer's disease.Neurosci Biobehav Rev. 2016 Jun;65:326-40. doi: 10.1016/j.neubiorev.2016.03.025. Epub 2016 Apr 1. Neurosci Biobehav Rev. 2016. PMID: 27044452 Free PMC article. Review.
-
Shared cognitive and behavioral impairments in epilepsy and Alzheimer's disease and potential underlying mechanisms.Epilepsy Behav. 2013 Mar;26(3):343-51. doi: 10.1016/j.yebeh.2012.11.040. Epub 2013 Jan 13. Epilepsy Behav. 2013. PMID: 23321057 Free PMC article. Review.
-
Modeling protein-protein interactions in axon initial segment to understand their potential impact on action potential initiation.Neural Regen Res. 2021 Apr;16(4):700-706. doi: 10.4103/1673-5374.295332. Neural Regen Res. 2021. PMID: 33063731 Free PMC article.
-
Structure-activity relationship of memapsin 2: implications on physiological functions and Alzheimer's disease.Acta Biochim Biophys Sin (Shanghai). 2013 Aug;45(8):613-21. doi: 10.1093/abbs/gmt050. Epub 2013 May 15. Acta Biochim Biophys Sin (Shanghai). 2013. PMID: 23676825 Free PMC article. Review.
-
Sodium channel β2 subunit promotes filopodia-like processes and expansion of the dendritic tree in developing rat hippocampal neurons.Front Cell Neurosci. 2013 Jan 25;7:2. doi: 10.3389/fncel.2013.00002. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23355803 Free PMC article.
References
-
- Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr. Opin. Neurol. 2002;15:445–450. - PubMed
-
- Mendez M, Lim G. Seizures in elderly patients with dementia: epidemiology and management. Drugs Aging. 2003;20:791–803. - PubMed
-
- Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46:727–730. - PubMed
-
- Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology. 1986;36:1226–1230. - PubMed
-
- Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J. Neurol. 2006;253:139–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

