Role of hypoxia-inducible transcription factors 1alpha and 2alpha in the regulation of plasminogen activator inhibitor-1 expression in a human trophoblast cell line
- PMID: 17570486
- PMCID: PMC2001228
- DOI: 10.1016/j.placenta.2007.04.005
Role of hypoxia-inducible transcription factors 1alpha and 2alpha in the regulation of plasminogen activator inhibitor-1 expression in a human trophoblast cell line
Abstract
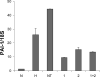
The plasminogen activator inhibitors (PAIs) play critical roles in regulating hemostatic and invasive functions of trophoblasts through suppression of plasmin-dependent fibrinolysis and extracellular matrix degradation. The expression of PAI-1 is increased under hypoxic conditions, although the mechanism remains incompletely understood. In the current study we used HTR-8/SVneo cells, a first trimester extravillous trophoblast cell line, and siRNA technology to examine the role of hypoxia-inducible transcription factors (HIFs)-1alpha and -2alpha in the regulation of PAI-1 expression. Using serum-containing and serum-free media culture media it was initially noted that levels of PAI-1, but not PAI-2 protein, were markedly induced by hypoxic (2-3% oxygen) treatment. Under hypoxic conditions, Western blotting revealed that the presence of siRNAs to HIF-1alpha and HIF-2alpha suppressed expression of their respective proteins, whereas treatment with non-targeting and cyclophilin B siRNAs did not. Importantly, incubation with siRNA to HIF-1alpha or HIF-2alpha alone reduced PAI-1 protein levels to a similar extent, with the combined treatment inducing a more profound effect. The presence of HIF siRNAs reduced levels of PAI-1 mRNA as measured by quantitative real-time PCR, indicating that HIF-1alpha and HIF-2 alpha regulate PAI-1 expression at a transcriptional level. These results indicate that both HIF-1alpha and HIF-2alpha play important and similar roles in hypoxia-mediated stimulation of PAI-1 expression in HTR-8/SVneo cells. Our findings provide insight into the physiological regulation of trophoblast PAI-1 expression in early pregnancy when placental oxygen levels are low, as well as a mechanism for over-expression of placental PAI-1 noted in pregnancies with preeclampsia.
Figures





Similar articles
-
Hypoxia induced HIF-1/HIF-2 activity alters trophoblast transcriptional regulation and promotes invasion.Eur J Cell Biol. 2015 Dec;94(12):589-602. doi: 10.1016/j.ejcb.2015.10.004. Epub 2015 Oct 24. Eur J Cell Biol. 2015. PMID: 26531845
-
HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts.Sci Rep. 2018 Nov 26;8(1):17375. doi: 10.1038/s41598-018-35745-1. Sci Rep. 2018. PMID: 30478339 Free PMC article.
-
Connexin 32 down-regulates the fibrinolytic factors in metastatic renal cell carcinoma cells.Life Sci. 2006 Apr 4;78(19):2249-54. doi: 10.1016/j.lfs.2005.09.036. Epub 2005 Nov 11. Life Sci. 2006. PMID: 16289236
-
Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review.Placenta. 2002 Apr;23 Suppl A:S47-57. doi: 10.1053/plac.2002.0815. Placenta. 2002. PMID: 11978059 Review.
-
Role of HIF-1α and HIF-2α in osteoarthritis.Joint Bone Spine. 2015 May;82(3):144-7. doi: 10.1016/j.jbspin.2014.10.003. Epub 2014 Dec 29. Joint Bone Spine. 2015. PMID: 25553838 Review.
Cited by
-
Changes of new coagulation markers in healthy pregnant women and establishment of reference intervals in Changsha.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 Apr 28;47(4):469-478. doi: 10.11817/j.issn.1672-7347.2022.210536. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35545342 Free PMC article.
-
The Role of Catestatin in Preeclampsia.Int J Mol Sci. 2024 Feb 20;25(5):2461. doi: 10.3390/ijms25052461. Int J Mol Sci. 2024. PMID: 38473713 Free PMC article. Review.
-
Oncostatin M, Serpins, and Oxidative Stress in Extracellular Matrix Remodeling and Arteriovenous Fistula Maturation.Cardiol Cardiovasc Med. 2023;7(2):129-140. doi: 10.26502/fccm.92920318. Epub 2023 Apr 20. Cardiol Cardiovasc Med. 2023. PMID: 37484520 Free PMC article.
-
Epsilon-aminocaproic acid prevents high glucose and insulin induced-invasiveness in MDA-MB-231 breast cancer cells, modulating the plasminogen activator system.Mol Cell Biochem. 2018 Jan;437(1-2):65-80. doi: 10.1007/s11010-017-3096-8. Epub 2017 Jun 13. Mol Cell Biochem. 2018. PMID: 28612231
-
Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11459-64. doi: 10.1073/pnas.1002443107. Epub 2010 Jun 7. Proc Natl Acad Sci U S A. 2010. PMID: 20534544 Free PMC article.
References
-
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. - PubMed
-
- Zini JM, Murray SC, Graham CH, Lala PK, Kariko K, Barnathan ES, Mazar A, Henkin J, Cines DB, McCrae KR. Characterization of urokinase receptor expression by human placental trophoblasts. Blood. 1992;79:2917–2929. - PubMed
-
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

