PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling
- PMID: 17557799
- PMCID: PMC2846428
- DOI: 10.1152/ajplung.00489.2006
PGE(2) inhibition of TGF-beta1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling
Abstract
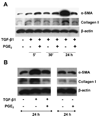
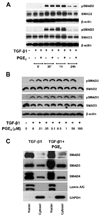
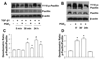
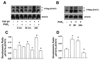
Myofibroblasts are pathogenic in pulmonary fibrotic disease due to their exuberant production of matrix rich in collagen that interferes with gas exchange and the ability of these cells to contract and distort the alveolar space. Transforming growth factor-beta1 (TGF-beta1) is a well-known inducer of myofibroblast differentiation. TGF-beta1-induced transformation of fibroblasts to apoptosis-resistant myofibroblasts is adhesion-dependent and focal adhesion kinase (FAK)-mediated. Prostaglandin E(2) (PGE(2)) inhibits this differentiation via E prostanoid receptor 2 (EP2) signaling and cAMP elevation, but whether PGE(2) does so by interfering with TGF-beta1 signaling is unknown. Thus we examined the effects of PGE(2) in the presence and absence of TGF-beta1 stimulation on candidate signaling pathways in human lung fibroblasts. We now demonstrate that PGE(2) does not interfere with TGF-beta1-induced Smad phosphorylation or its translocation to the nucleus. Rather, PGE(2) has dramatic effects on cell shape and cytoskeletal architecture and disrupts the formation of appropriate focal adhesions. PGE(2) treatment diminishes TGF-beta1-induced phosphorylation of paxillin, STAT-3, and FAK and, in turn, limits activation of the protein kinase B (PKB/Akt) pathway. These alterations do not, however, result in increased apoptosis within the first 24 h of treatment. Interestingly, the effects of PGE(2) stimulation alone do not always mirror the effects of PGE(2) in the presence of TGF-beta1, indicating that the context for EP2 signaling is different in the presence of TGF-beta1. Taken together, our results demonstrate that PGE(2) has the potential to limit TGF-beta1-induced myofibroblast differentiation via adhesion-dependent, but Smad-independent, pathways.
Figures


Similar articles
-
Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase.J Biol Chem. 2003 Apr 4;278(14):12384-9. doi: 10.1074/jbc.M208544200. Epub 2003 Jan 16. J Biol Chem. 2003. PMID: 12531888
-
Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts.Cell Signal. 2007 Apr;19(4):761-71. doi: 10.1016/j.cellsig.2006.10.001. Epub 2006 Nov 17. Cell Signal. 2007. PMID: 17113264 Free PMC article.
-
Focal adhesion kinase signaling determines the fate of lung epithelial cells in response to TGF-β.Am J Physiol Lung Cell Mol Physiol. 2017 Jun 1;312(6):L926-L935. doi: 10.1152/ajplung.00121.2016. Epub 2017 Mar 30. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28360109 Free PMC article.
-
Prostaglandin E2 and the pathogenesis of pulmonary fibrosis.Am J Respir Cell Mol Biol. 2011 Sep;45(3):445-52. doi: 10.1165/rcmb.2011-0025RT. Epub 2011 Mar 18. Am J Respir Cell Mol Biol. 2011. PMID: 21421906 Free PMC article. Review.
-
SnoN as a novel negative regulator of TGF-β/Smad signaling: a target for tailoring organ fibrosis.Am J Physiol Heart Circ Physiol. 2015 Jan 15;308(2):H75-82. doi: 10.1152/ajpheart.00453.2014. Epub 2014 Nov 7. Am J Physiol Heart Circ Physiol. 2015. PMID: 25380815 Review.
Cited by
-
Lipid Metabolism in Tumor-Associated Fibroblasts.Adv Exp Med Biol. 2021;1316:117-131. doi: 10.1007/978-981-33-6785-2_8. Adv Exp Med Biol. 2021. PMID: 33740247 Review.
-
Inhibition of miR-29 by TGF-beta-Smad3 signaling through dual mechanisms promotes transdifferentiation of mouse myoblasts into myofibroblasts.PLoS One. 2012;7(3):e33766. doi: 10.1371/journal.pone.0033766. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438993 Free PMC article.
-
Phosphatase and tensin homologue on chromosome 10 (PTEN) directs prostaglandin E2-mediated fibroblast responses via regulation of E prostanoid 2 receptor expression.J Biol Chem. 2009 Nov 20;284(47):32264-71. doi: 10.1074/jbc.M109.004796. Epub 2009 Oct 6. J Biol Chem. 2009. PMID: 19808686 Free PMC article.
-
Identifying mechanisms driving formation of granuloma-associated fibrosis during Mycobacterium tuberculosis infection.J Theor Biol. 2017 Sep 21;429:1-17. doi: 10.1016/j.jtbi.2017.06.017. Epub 2017 Jun 20. J Theor Biol. 2017. PMID: 28642013 Free PMC article.
-
The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair.J Int Oral Health. 2015 Mar;7(3):75-80. J Int Oral Health. 2015. PMID: 25878485 Free PMC article. Review.
References
-
- Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G, Trojanowska M, Gilliam AC, Varga J. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor beta. Arthritis Rheum. 2005;52:1248–1258. - PubMed
-
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. - PubMed
-
- Bulin C, Albrecht U, Bode JG, Weber AA, Schror K, Levkau B, Fischer JW. Differential effects of vasodilatory prostaglandins on focal adhesions, cytoskeletal architecture, and migration in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:84–89. - PubMed
-
- Charbeneau RP, Christensen PJ, Chrisman CJ, Paine R, Toews GB, Peters-Golden M, Moore BB. Impaired synthesis of prostaglandin E2 by lung fibroblasts and alveolar epithelial cells from GM-CSF−/−mice: implications for fibroproliferation. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1103–L1111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

