Ab initio modeling of glycosyl torsions and anomeric effects in a model carbohydrate: 2-ethoxy tetrahydropyran
- PMID: 17554075
- PMCID: PMC1914444
- DOI: 10.1529/biophysj.106.099986
Ab initio modeling of glycosyl torsions and anomeric effects in a model carbohydrate: 2-ethoxy tetrahydropyran
Abstract
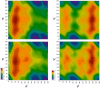
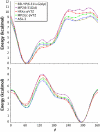
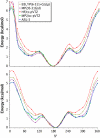
A range of ab initio calculations were carried out on the axial and equatorial anomers of the model carbohydrate 2-ethoxy tetrahydropyran to evaluate the level of theory required to accurately evaluate the glycosyl dihedral angle and the anomeric ratio. Vacuum CCSD(T)/CBS extrapolations at the global minimum yield DeltaE = E(equatorial) - E(axial) = 1.42 kcal/mol. When corrected for solvent (by the IEFPCM model), zero-point vibrations and entropy, DeltaG(298) = 0.49 kcal/mol, in excellent agreement with the experimental value of 0.47 +/- 0.3 kcal/mol. A new additivity scheme, the layered composite method (LCM), yields DeltaE to within 0.1 kcal/mol of the CCSD(T)/CBS result at a fraction of the computer requirements. Anomeric ratios and one-dimensional torsional surfaces generated by LCM and the even more efficient MP2/cc-pVTZ level of theory are in excellent agreement, indicating that the latter is suitable for force-field parameterization of carbohydrates. Hartree-Fock and density functional theory differ from CCSD(T)/CBS for DeltaE by approximately 1 kcal/mol; they show similar deviations in torsional surfaces evaluated from LCM. A comparison of vacuum and solvent-corrected one- and two-dimensional torsional surfaces indicates the equatorial form of 2-ethoxy tetrahydropyran is more sensitive to solvent than the axial.
Figures
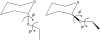
Similar articles
-
Intrinsic conformational preferences of substituted cyclohexanes and tetrahydropyrans evaluated at the CCSD(T) complete basis set limit: implications for the anomeric effect.J Phys Chem A. 2005 Dec 8;109(48):11073-9. doi: 10.1021/jp0550311. J Phys Chem A. 2005. PMID: 16331953
-
Benchmark theoretical study of the π-π binding energy in the benzene dimer.J Phys Chem A. 2014 Sep 4;118(35):7568-78. doi: 10.1021/jp5024235. Epub 2014 May 5. J Phys Chem A. 2014. PMID: 24761749
-
Conformational properties of 1-silyl-1-silacyclohexane, C(5)H(10)SiHSiH(3): gas electron diffraction, low-temperature NMR, temperature-dependent Raman spectroscopy, and quantum chemical calculations (&).J Phys Chem A. 2010 Feb 11;114(5):2127-35. doi: 10.1021/jp909942u. J Phys Chem A. 2010. PMID: 20073516
-
Derivation of class II force fields. VI. Carbohydrate compounds and anomeric effects.Biopolymers. 1998 May;45(6):435-68. doi: 10.1002/(SICI)1097-0282(199805)45:6<435::AID-BIP3>3.0.CO;2-Q. Biopolymers. 1998. PMID: 9538697 Review.
-
Calculations on noncovalent interactions and databases of benchmark interaction energies.Acc Chem Res. 2012 Apr 17;45(4):663-72. doi: 10.1021/ar200255p. Epub 2012 Jan 6. Acc Chem Res. 2012. PMID: 22225511 Review.
Cited by
-
Recent synthetic approaches toward non-anomeric spiroketals in natural products.Molecules. 2008;13(10):2570-600. doi: 10.3390/molecules13102570. Molecules. 2008. PMID: 18927519 Free PMC article. Review.
-
CHARMM Additive All-Atom Force Field for Glycosidic Linkages between Hexopyranoses.J Chem Theory Comput. 2009 Aug 20;5(9):2353-2370. doi: 10.1021/ct900242e. J Chem Theory Comput. 2009. PMID: 20161005 Free PMC article.
-
CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields.J Comput Chem. 2010 Mar;31(4):671-90. doi: 10.1002/jcc.21367. J Comput Chem. 2010. PMID: 19575467 Free PMC article.
-
CHARMM additive all-atom force field for aldopentofuranoses, methyl-aldopentofuranosides, and fructofuranose.J Phys Chem B. 2009 Sep 17;113(37):12466-76. doi: 10.1021/jp905496e. J Phys Chem B. 2009. PMID: 19694450 Free PMC article.
-
Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types.J Phys Chem B. 2010 Jun 17;114(23):7830-43. doi: 10.1021/jp101759q. J Phys Chem B. 2010. PMID: 20496934 Free PMC article.
References
-
- Rao, V. S. R., P. K. Quasba, P. V. Balaji, and R. Chandrasekaran. 1998. Conformation of Carbohydrates. Harwood, Amsterdam, The Netherlands.
-
- Brady, J. W., and R. K. Schmidt. 1993. The role of hydrogen-bonding in carbohydrates—molecular dynamics simulations of maltose in aqueous solution. J. Phys. Chem. 97:958–966.
-
- Metzler, D. E. 2001. Biochemistry, 2nd Ed. Harcourt Academic Press, San Diego, CA.
-
- Edward, J. T. 1955. Stability of glycosides to acid hydrolysis. Chem. Ind. (London). 36:1102–1104.
-
- Lemieux, R. U., and P. Chu. 1958. Abstracts of Papers. 133rd National Meeting of the American Chemical Society, San Francisco, CA. American Chemical Society, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

