Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling
- PMID: 17553962
- PMCID: PMC1888574
- DOI: 10.1073/pnas.0703004104
Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling
Abstract
During the hair cycle, follicle stem cells (SCs) residing in a specialized niche called the "bulge" undergo bouts of quiescence and activation to cyclically regenerate new hairs. Developmental studies have long implicated the canonical bone morphogenetic protein (BMP) pathway in hair follicle (HF) determination and differentiation, but how BMP signaling functions in the hair follicle SC niche remains unknown. Here, we use loss and gain of function studies to manipulate BMP signaling in the SC niche. We show that when the Bmpr1a gene is conditionally ablated, otherwise quiescent SCs are activated to proliferate, causing an expansion of the niche and loss of slow-cycling cells. Surprisingly, follicle SCs are not lost, however, but rather, they generate long-lived, tumor-like branches that express Sox4, Lhx2, and Sonic Hedgehog but fail to terminally differentiate to make hair. A key component of BMPR1A-deficient SCs is their elevated levels of both Lef1 and beta-catenin, which form a bipartite transcription complex required for initiation of the hair cycle. Although beta-catenin can be stabilized by Wnt signaling, we show that BMPR1A deficiency enhances beta-catenin stabilization in the niche through a pathway involving PTEN inhibition and PI3K/AKT activation. Conversely, sustained BMP signaling in the SC niche blocks activation and promotes premature hair follicle differentiation. Together, these studies reveal the importance of balancing BMP signaling in the SC niche.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
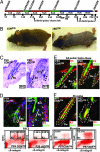
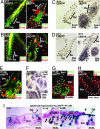

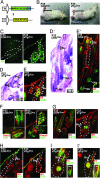
Similar articles
-
Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion.Stem Cells. 2006 Dec;24(12):2826-39. doi: 10.1634/stemcells.2005-0544. Epub 2006 Sep 7. Stem Cells. 2006. PMID: 16960130
-
Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA.J Cell Biol. 2003 Nov 10;163(3):609-23. doi: 10.1083/jcb.200309042. J Cell Biol. 2003. PMID: 14610062 Free PMC article.
-
Unveiling hair follicle stem cells.Stem Cell Rev Rep. 2010 Dec;6(4):658-64. doi: 10.1007/s12015-010-9172-z. Stem Cell Rev Rep. 2010. PMID: 20676942 Review.
-
Wnt7b is an important intrinsic regulator of hair follicle stem cell homeostasis and hair follicle cycling.Stem Cells. 2014 Apr;32(4):886-901. doi: 10.1002/stem.1599. Stem Cells. 2014. PMID: 24222445 Free PMC article.
-
Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling.Curr Opin Genet Dev. 2001 Oct;11(5):541-6. doi: 10.1016/s0959-437x(00)00230-6. Curr Opin Genet Dev. 2001. PMID: 11532396 Review.
Cited by
-
Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation.Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):1351-6. doi: 10.1073/pnas.1121312110. Epub 2013 Jan 4. Proc Natl Acad Sci U S A. 2013. PMID: 23292934 Free PMC article.
-
Constitutively active Akt induces ectodermal defects and impaired bone morphogenetic protein signaling.Mol Biol Cell. 2008 Jan;19(1):137-49. doi: 10.1091/mbc.e07-08-0764. Epub 2007 Oct 24. Mol Biol Cell. 2008. PMID: 17959825 Free PMC article.
-
Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells.Stem Cell Res. 2014 Mar;12(2):562-73. doi: 10.1016/j.scr.2014.01.003. Epub 2014 Jan 22. Stem Cell Res. 2014. PMID: 24530980 Free PMC article.
-
Akt2 and SGK3 are both determinants of postnatal hair follicle development.FASEB J. 2009 Sep;23(9):3193-202. doi: 10.1096/fj.08-123729. Epub 2009 May 11. FASEB J. 2009. PMID: 19433625 Free PMC article.
-
PTEN, stem cells, and cancer stem cells.J Biol Chem. 2009 May 1;284(18):11755-9. doi: 10.1074/jbc.R800071200. Epub 2008 Dec 30. J Biol Chem. 2009. PMID: 19117948 Free PMC article. Review.
References
-
- Moore KA, Lemischka IR. Science. 2006;311:1880–1885. - PubMed
-
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Cell. 2000;102:451–461. - PubMed
-
- Panteleyev AA, Jahoda CA, Christiano AM. J Cell Sci. 2001;114:3419–3431. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Cell. 2004;118:635–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

