Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells
- PMID: 17553885
- PMCID: PMC1951378
- DOI: 10.1128/JVI.00228-07
Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells
Abstract
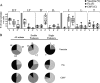
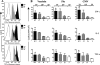
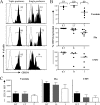
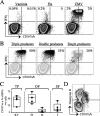
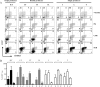
Virus-specific CD4 T cells are endowed with multiple functions, such as cytokine production, CD40 ligand (CD40L) expression (associated with the costimulation of CD8 and B cells), and degranulation (associated with cytotoxic potential). Here, we used antiviral CD4 T cells present in human blood to evaluate the relationship between cytokine production and other functions of CD4 T cells. Antiviral CD4 T cells specific for a virus causing persistent infection, cytomegalovirus (CMV), and two viruses causing nonpersistent infections, influenza virus and the smallpox vaccine virus (vaccinia virus), were studied. CD4 T cells specific for each of the viruses produced all seven possible combinations of the cytokines gamma interferon (IFN-gamma), interleukin-2, and tumor necrosis factor alpha. Cells producing three or two cytokines (triple producers and double producers) represented nearly 50% of the total response to each of the viruses. Triple producers expressed the highest levels of cytokines per cell, and single producers expressed the lowest. Following stimulation, higher frequencies of triple producers than single producers expressed CD40L. Only CMV-specific CD4 T cells underwent degranulation. However, higher frequencies of CMV-specific triple producers than single producers showed this functional characteristic. In contrast to the functional phenotypes, the memory phenotypes of triple producers and IFN-gamma single producers did not differ. These results demonstrate a strong positive association between the cytokine coproduction capacity of a virus-specific CD4 T cell and its other functional characteristics and suggest that vaccines should aim to elicit T cells that coproduce more than one cytokine.
Figures
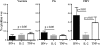
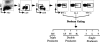
Similar articles
-
Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines.J Virol. 2007 Nov;81(21):12071-6. doi: 10.1128/JVI.01261-07. Epub 2007 Aug 29. J Virol. 2007. PMID: 17728221 Free PMC article.
-
[Monitoring of cytomegalovirus-specific CD4+ and CD8+ T cell responses by cytokine flow cytometry in renal transplant recipients].Mikrobiyol Bul. 2016 Apr;50(2):224-35. Mikrobiyol Bul. 2016. PMID: 27175495 Turkish.
-
Determination of cytokine co-expression in individual splenic CD4+ and CD8+ T cells from influenza virus-immune mice.Immunology. 1998 Nov;95(3):346-51. doi: 10.1046/j.1365-2567.1998.00608.x. Immunology. 1998. PMID: 9824496 Free PMC article.
-
Analyzing T-cell responses to cytomegalovirus by cytokine flow cytometry.Hum Immunol. 2004 May;65(5):493-9. doi: 10.1016/j.humimm.2004.02.004. Hum Immunol. 2004. PMID: 15172449 Review.
-
Induction and function of virus-specific CD4+ T cell responses.Virology. 2011 Mar 15;411(2):216-28. doi: 10.1016/j.virol.2010.12.015. Epub 2011 Jan 14. Virology. 2011. PMID: 21236461 Free PMC article. Review.
Cited by
-
ChAdOx1-vectored Lassa fever vaccine elicits a robust cellular and humoral immune response and protects guinea pigs against lethal Lassa virus challenge.NPJ Vaccines. 2021 Mar 2;6(1):32. doi: 10.1038/s41541-021-00291-x. NPJ Vaccines. 2021. PMID: 33654106 Free PMC article.
-
HIV therapeutic vaccines: moving towards a functional cure.Curr Opin Immunol. 2015 Aug;35:1-8. doi: 10.1016/j.coi.2015.05.001. Epub 2015 May 18. Curr Opin Immunol. 2015. PMID: 25996629 Free PMC article. Review.
-
O-mannosylation of the Mycobacterium tuberculosis adhesin Apa is crucial for T cell antigenicity during infection but is expendable for protection.PLoS Pathog. 2013;9(10):e1003705. doi: 10.1371/journal.ppat.1003705. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130497 Free PMC article. Clinical Trial.
-
Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process.Immunology. 2011 Mar;132(3):326-39. doi: 10.1111/j.1365-2567.2010.03386.x. Epub 2011 Jan 7. Immunology. 2011. PMID: 21214539 Free PMC article.
-
Tuberculosis vaccine with high predicted population coverage and compatibility with modern diagnostics.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):1096-101. doi: 10.1073/pnas.1314973111. Epub 2014 Jan 6. Proc Natl Acad Sci U S A. 2014. PMID: 24395772 Free PMC article.
References
-
- Amara, R. R., J. M. Smith, S. Staprans, D. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. - PubMed
-
- Blattman, J. N., J. M. Grayson, E. J. Wherry, S. M. Kaech, K. A. Smith, and R. Ahmed. 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9:540-547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

