Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1
- PMID: 17550303
- PMCID: PMC1885450
- DOI: 10.1371/journal.pmed.0040186
Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1
Abstract
Background: While vascular endothelial growth factor (VEGF) expression in breast tumors has been correlated with a poor outcome in the pathogenesis of breast cancer, the expression, localization, and function of VEGF receptors VEGFR1 (also known as FLT1) and VEGFR2 (also known as KDR or FLK1), as well as neuropilin 1 (NRP1), in breast cancer are controversial.
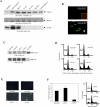
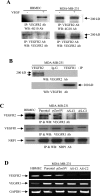
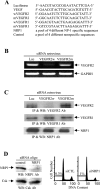
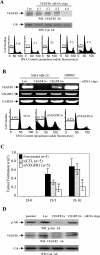
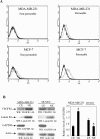
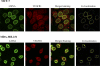
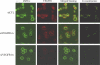
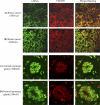
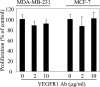
Methods and findings: We investigated the expression and function of VEGF and VEGF receptors in breast cancer cells. We observed that VEGFR1 expression was abundant, VEGFR2 expression was low, and NRP1 expression was variable. MDA-MB-231 and MCF-7 breast cancer cells, transfected with antisense VEGF cDNA or with siVEGF (VEGF-targeted small interfering RNA), showed a significant reduction in VEGF expression and increased apoptosis as compared to the control cells. Additionally, specifically targeted knockdown of VEGFR1 expression by siRNA (siVEGFR1) significantly decreased the survival of breast cancer cells through down-regulation of protein kinase B (AKT) phosphorylation, while targeted knockdown of VEGFR2 or NRP1 expression had no effect on the survival of these cancer cells. Since a VEGFR1-specific ligand, placenta growth factor (PGF), did not, as expected, inhibit the breast cancer cell apoptosis induced by siVEGF, and since VEGFR1 antibody also had no effects on the survival of these cells, we examined VEGFR1 localization. VEGFR1 was predominantly expressed internally in MDA-MB-231 and MCF-7 breast cancer cells. Specifically, VEGFR1 was found to be colocalized with lamin A/C and was expressed mainly in the nuclear envelope in breast cancer cell lines and primary breast cancer tumors. Breast cancer cells treated with siVEGFR1 showed significantly decreased VEGFR1 expression levels and a lack of VEGFR1 expression in the nuclear envelope.
Conclusions: This study provides, to our knowledge for the first time, evidence of a unique survival system in breast cancer cells by which VEGF can act as an internal autocrine (intracrine) survival factor through its binding to VEGFR1. These results may lead to an improved strategy for tumor therapy based on the inhibition of angiogenesis.
Conflict of interest statement
Figures

Similar articles
-
Anti-flt1 peptide, a vascular endothelial growth factor receptor 1-specific hexapeptide, inhibits tumor growth and metastasis.Clin Cancer Res. 2005 Apr 1;11(7):2651-61. doi: 10.1158/1078-0432.CCR-04-1564. Clin Cancer Res. 2005. PMID: 15814646
-
Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver.J Natl Cancer Inst. 2008 Jan 16;100(2):109-20. doi: 10.1093/jnci/djm279. Epub 2008 Jan 8. J Natl Cancer Inst. 2008. PMID: 18182619
-
VEGF-A165 induces human aortic smooth muscle cell migration by activating neuropilin-1-VEGFR1-PI3K axis.Biochemistry. 2008 Mar 18;47(11):3345-51. doi: 10.1021/bi8000352. Epub 2008 Feb 20. Biochemistry. 2008. PMID: 18284215
-
Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis.J Biochem Mol Biol. 2006 Sep 30;39(5):469-78. doi: 10.5483/bmbrep.2006.39.5.469. J Biochem Mol Biol. 2006. PMID: 17002866 Review.
-
Effects of vascular endothelial growth factors and their receptors on megakaryocytes and platelets and related diseases.Br J Haematol. 2018 Feb;180(3):321-334. doi: 10.1111/bjh.15000. Epub 2017 Oct 26. Br J Haematol. 2018. PMID: 29076133 Review.
Cited by
-
Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone.Invest Ophthalmol Vis Sci. 2012 Apr 30;53(4):2328-36. doi: 10.1167/iovs.11-8393. Invest Ophthalmol Vis Sci. 2012. PMID: 22427553 Free PMC article.
-
Placental growth factor (PlGF) enhances breast cancer cell motility by mobilising ERK1/2 phosphorylation and cytoskeletal rearrangement.Br J Cancer. 2010 Jun 29;103(1):82-9. doi: 10.1038/sj.bjc.6605746. Epub 2010 Jun 15. Br J Cancer. 2010. PMID: 20551949 Free PMC article.
-
Dynamic gene regulation by nuclear colony-stimulating factor 1 receptor in human monocytes and macrophages.Nat Commun. 2019 Apr 26;10(1):1935. doi: 10.1038/s41467-019-09970-9. Nat Commun. 2019. PMID: 31028249 Free PMC article.
-
Suppression of the ERK-SRF axis facilitates somatic cell reprogramming.Exp Mol Med. 2018 Feb 23;50(2):e448. doi: 10.1038/emm.2017.279. Exp Mol Med. 2018. PMID: 29472703 Free PMC article.
-
Trafficking of receptor tyrosine kinases to the nucleus.Exp Cell Res. 2009 May 15;315(9):1556-66. doi: 10.1016/j.yexcr.2008.09.027. Epub 2008 Oct 11. Exp Cell Res. 2009. PMID: 18951890 Free PMC article. Review.
References
-
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2004;23:1011–1027. - PubMed
-
- Cai J, Ahmad S, Jiang WG, Huang J, Kontos CD, et al. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003;52:2959–2968. - PubMed
-
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. - PubMed
-
- Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87:506–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

