Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses
- PMID: 17548520
- PMCID: PMC2118606
- DOI: 10.1084/jem.20062694
Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses
Abstract
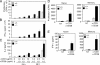
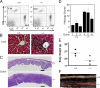
The deubiquitinating enzyme CYLD has recently been implicated in the regulation of signal transduction, but its physiological function and mechanism of action are still elusive. In this study, we show that CYLD plays a pivotal role in regulating T cell activation and homeostasis. T cells derived from Cyld knockout mice display a hyperresponsive phenotype and mediate the spontaneous development of intestinal inflammation. Interestingly, CYLD targets a ubiquitin-dependent kinase, transforming growth factor-beta-activated kinase 1 (Tak1), and inhibits its ubiquitination and autoactivation. Cyld-deficient T cells exhibit constitutively active Tak1 and its downstream kinases c-Jun N-terminal kinase and IkappaB kinase beta. These results emphasize a critical role for CYLD in preventing spontaneous activation of the Tak1 axis of T cell signaling and, thereby, maintaining normal T cell function.
Figures



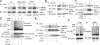
Similar articles
-
PKCθ/β and CYLD are antagonistic partners in the NFκB and NFAT transactivation pathways in primary mouse CD3+ T lymphocytes.PLoS One. 2013;8(1):e53709. doi: 10.1371/journal.pone.0053709. Epub 2013 Jan 15. PLoS One. 2013. PMID: 23335970 Free PMC article.
-
Differential requirement of IKK2 for CYLD-dependent representation of thymic and peripheral T-cell populations.Eur J Immunol. 2011 Oct;41(10):3054-62. doi: 10.1002/eji.201041160. Epub 2011 Aug 30. Eur J Immunol. 2011. PMID: 21728169
-
The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor β (TGF-β) signaling and the development of regulatory T cells.J Biol Chem. 2011 Nov 25;286(47):40520-30. doi: 10.1074/jbc.M111.292961. Epub 2011 Sep 19. J Biol Chem. 2011. PMID: 21931165 Free PMC article.
-
Deubiquitinating enzyme CYLD regulates the peripheral development and naive phenotype maintenance of B cells.J Biol Chem. 2007 May 25;282(21):15884-93. doi: 10.1074/jbc.M609952200. Epub 2007 Mar 27. J Biol Chem. 2007. PMID: 17392286
-
Ubiquitin-mediated activation of TAK1 and IKK.Oncogene. 2007 May 14;26(22):3214-26. doi: 10.1038/sj.onc.1210413. Oncogene. 2007. PMID: 17496917 Review.
Cited by
-
Deubiquitinating Enzyme: A Potential Secondary Checkpoint of Cancer Immunity.Front Oncol. 2020 Aug 7;10:1289. doi: 10.3389/fonc.2020.01289. eCollection 2020. Front Oncol. 2020. PMID: 32850399 Free PMC article. Review.
-
Forced expression of the non-coding RNA miR-17∼92 restores activation and function in CD28-deficient CD4+ T cells.iScience. 2022 Oct 17;25(11):105372. doi: 10.1016/j.isci.2022.105372. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388982 Free PMC article.
-
PKCθ/β and CYLD are antagonistic partners in the NFκB and NFAT transactivation pathways in primary mouse CD3+ T lymphocytes.PLoS One. 2013;8(1):e53709. doi: 10.1371/journal.pone.0053709. Epub 2013 Jan 15. PLoS One. 2013. PMID: 23335970 Free PMC article.
-
Synergistic and feedback signaling mechanisms in the regulation of inflammation in respiratory infections.Cell Mol Immunol. 2012 Mar;9(2):131-5. doi: 10.1038/cmi.2011.65. Epub 2012 Feb 6. Cell Mol Immunol. 2012. PMID: 22307042 Free PMC article. Review.
-
Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination.EMBO Rep. 2009 Nov;10(11):1242-9. doi: 10.1038/embor.2009.210. Epub 2009 Oct 9. EMBO Rep. 2009. PMID: 19820695 Free PMC article.
References
-
- Siegel, R.M., M. Katsumata, S. Komori, S. Wadsworth, L. Gill-Morse, S. Jerrold-Jones, A. Bhandoola, M.I. Greene, and K. Yui. 1990. Mechanisms of autoimmunity in the context of T-cell tolerance: insights from natural and transgenic animal model systems. Immunol. Rev. 118:165–192. - PubMed
-
- Liu, Y.C. 2004. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 22:81–127. - PubMed
-
- Wilkinson, K.D. 2000. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol. 11:141–148. - PubMed
-
- Reiley, W.W., M. Zhang, W. Jin, M. Losiewicz, K.B. Donohue, C.C. Norbury, and S.C. Sun. 2006. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat. Immunol. 7:411–417. - PubMed
-
- Bignell, G.R., W. Warren, S. Seal, M. Takahashi, E. Rapley, R. Barfoot, H. Green, C. Brown, P.J. Biggs, S.R. Lakhani, et al. 2000. Identification of the familial cylindromatosis tumour-suppressor gene. Nat. Genet. 25:160–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI064639/AI/NIAID NIH HHS/United States
- C06 RR-15428-01/RR/NCRR NIH HHS/United States
- CA94922/CA/NCI NIH HHS/United States
- AI057555/AI/NIAID NIH HHS/United States
- R56 AI056094/AI/NIAID NIH HHS/United States
- 2T32CA60395-11/CA/NCI NIH HHS/United States
- AI064639/AI/NIAID NIH HHS/United States
- R01 AI057555/AI/NIAID NIH HHS/United States
- T32 CA060395/CA/NCI NIH HHS/United States
- R01 CA094922/CA/NCI NIH HHS/United States
- R01 AI056094/AI/NIAID NIH HHS/United States
- AI056094/AI/NIAID NIH HHS/United States
- R37 AI064639/AI/NIAID NIH HHS/United States
- C06 RR015428/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

