Non-heme Fe(IV)-oxo intermediates
- PMID: 17542550
- PMCID: PMC3870002
- DOI: 10.1021/ar700066p
Non-heme Fe(IV)-oxo intermediates
Abstract
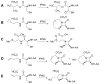
High-valent non-heme iron-oxo intermediates have been proposed for decades as the key intermediates in numerous biological oxidation reactions. In the past three years, the first direct characterization of such intermediates has been provided by studies of several alphaKG-dependent oxygenases that catalyze either hydroxylation or halogenation of their substrates. In each case, the Fe(IV)-oxo intermediate is implicated in cleavage of the aliphatic C-H bond to initiate hydroxylation or halogenation. The observation of non-heme Fe(IV)-oxo intermediates and Fe(II)-containing product(s) complexes with almost identical spectroscopic parameters in the reactions of two distantly related alphaKG-dependent hydroxylases suggests that members of this subfamily follow a conserved mechanism for substrate hydroxylation. In contrast, for the alphaKG-dependent non-heme iron halogenase, CytC3, two distinct Fe(IV) complexes form and decay together, suggesting that they are in rapid equilibrium. The existence of two distinct conformers of the Fe site may be the key factor accounting for the divergence of the halogenase reaction from the more usual hydroxylation pathway after C-H bond cleavage. Distinct transformations catalyzed by other mononuclear non-heme enzymes are likely also to involve initial C-H bond cleavage by Fe(IV)-oxo complexes, followed by diverging reactivities of the resulting Fe(III)-hydroxo/substrate radical intermediates.
Figures

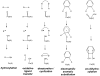
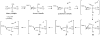
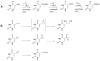
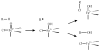
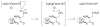

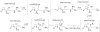
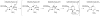
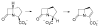
Similar articles
-
Stereospecific alkane hydroxylation by non-heme iron catalysts: mechanistic evidence for an Fe(V)=O active species.J Am Chem Soc. 2001 Jul 4;123(26):6327-37. doi: 10.1021/ja010310x. J Am Chem Soc. 2001. PMID: 11427057
-
Steady-state and transient kinetic analyses of taurine/alpha-ketoglutarate dioxygenase: effects of oxygen concentration, alternative sulfonates, and active-site variants on the FeIV-oxo intermediate.Biochemistry. 2005 Mar 15;44(10):3845-55. doi: 10.1021/bi048746n. Biochemistry. 2005. PMID: 15751960
-
The first direct characterization of a high-valent iron intermediate in the reaction of an alpha-ketoglutarate-dependent dioxygenase: a high-spin FeIV complex in taurine/alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli.Biochemistry. 2003 Jun 24;42(24):7497-508. doi: 10.1021/bi030011f. Biochemistry. 2003. PMID: 12809506
-
Catalytic Mechanisms of Fe(II)- and 2-Oxoglutarate-dependent Oxygenases.J Biol Chem. 2015 Aug 21;290(34):20702-20711. doi: 10.1074/jbc.R115.648691. Epub 2015 Jul 7. J Biol Chem. 2015. PMID: 26152721 Free PMC article. Review.
-
High-valent iron(IV)-oxo complexes of heme and non-heme ligands in oxygenation reactions.Acc Chem Res. 2007 Jul;40(7):522-31. doi: 10.1021/ar700027f. Epub 2007 May 1. Acc Chem Res. 2007. PMID: 17469792 Review.
Cited by
-
Mono- and binuclear non-heme iron chemistry from a theoretical perspective.J Biol Inorg Chem. 2016 Sep;21(5-6):619-44. doi: 10.1007/s00775-016-1357-8. Epub 2016 May 26. J Biol Inorg Chem. 2016. PMID: 27229513 Review.
-
Pathways of the Extremely Reactive Iron(IV)-oxido complexes with Tetradentate Bispidine Ligands.Chemistry. 2021 Aug 5;27(44):11377-11390. doi: 10.1002/chem.202101045. Epub 2021 Jul 5. Chemistry. 2021. PMID: 34121233 Free PMC article.
-
Dissecting the Mechanism of the Nonheme Iron Endoperoxidase FtmOx1 Using Substrate Analogues.JACS Au. 2022 Jun 10;2(7):1686-1698. doi: 10.1021/jacsau.2c00248. eCollection 2022 Jul 25. JACS Au. 2022. PMID: 35911443 Free PMC article.
-
Enzymatic Cascade Reactions in Biosynthesis.Angew Chem Int Ed Engl. 2019 May 20;58(21):6846-6879. doi: 10.1002/anie.201807844. Epub 2019 Feb 20. Angew Chem Int Ed Engl. 2019. PMID: 30156048 Free PMC article. Review.
-
Reactive Cobalt⁻Oxo Complexes of Tetrapyrrolic Macrocycles and N-based Ligand in Oxidative Transformation Reactions.Molecules. 2018 Dec 26;24(1):78. doi: 10.3390/molecules24010078. Molecules. 2018. PMID: 30587824 Free PMC article. Review.
References
-
- Costas M, Mehn MP, Jensen MP, Que L., Jr Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates. Chem Rev. 2004;104:939–986. - PubMed
-
- Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Geometric and Electronic Structure/Function Correlations in Non-Heme Iron Enzymes. Chem Rev. 2000;100:235–349. - PubMed
-
- Hausinger RP. Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39:21–68. - PubMed
-
- Vaillancourt FH, Yeh E, Vosburg DA, Garneau-Tsodikova S, Walsh CT. Nature’s Inventory of Halogenation Catalysts: Oxidative Strategies Predominate. Chem Rev. 2006;106:3364–3378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

