Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle
- PMID: 17540697
- PMCID: PMC2075270
- DOI: 10.1113/jphysiol.2007.136614
Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle
Abstract
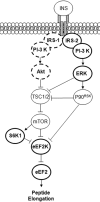
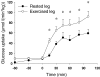
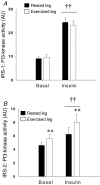
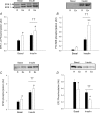
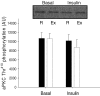
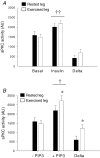
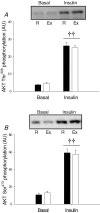
We investigated if acute endurance-type exercise interacts with insulin-stimulated activation of atypical protein kinase C (aPKC) and insulin signalling to peptide chain elongation in human skeletal muscle. Four hours after acute one-legged exercise, insulin-induced glucose uptake was approximately 80% higher (N = 12, P < 0.05) in previously exercised muscle, measured during a euglycaemic-hyperinsulinaemic clamp (100 microU ml(-1)). Insulin increased (P < 0.05) both insulin receptor substrate (IRS)-1 and IRS-2 associated phosphatidylinositol (PI)-3 kinase activity and led to increased (P < 0.001) phosphorylation of Akt on Ser(473) and Thr(308) in skeletal muscle. Interestingly, in response to prior exercise IRS-2-associated PI-3 kinase activity was higher (P < 0.05) both at basal and during insulin stimulation. This coincided with correspondingly altered phosphorylation of the extracellular-regulated protein kinase 1/2 (ERK 1/2), p70S6 kinase (P70S6K), eukaryotic elongation factor 2 (eEF2) kinase and eEF2. aPKC was similarly activated by insulin in rested and exercised muscle, without detectable changes in aPKC Thr(410) phosphorylation. However, when adding phosphatidylinositol-3,4,5-triphosphate (PIP3), the signalling product of PI-3 kinase, to basal muscle homogenates, aPKC was more potently activated (P = 0.01) in previously exercised muscle. Collectively, this study shows that endurance-type exercise interacts with insulin signalling to peptide chain elongation. Although protein turnover was not evaluated, this suggests that capacity for protein synthesis after acute endurance-type exercise may be improved. Furthermore, endurance exercise increased the responsiveness of aPKC to PIP3 providing a possible link to improved insulin-stimulated glucose uptake after exercise.
Figures
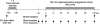
Similar articles
-
Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects.Diabetologia. 2006 Feb;49(2):375-82. doi: 10.1007/s00125-005-0112-4. Epub 2006 Jan 5. Diabetologia. 2006. PMID: 16395615 Clinical Trial.
-
Insulin signaling in human skeletal muscle: time course and effect of exercise.Diabetes. 1997 Nov;46(11):1775-81. doi: 10.2337/diab.46.11.1775. Diabetes. 1997. PMID: 9356025
-
Combined thiazolidinedione-metformin treatment synergistically improves insulin signalling to insulin receptor substrate-1-dependent phosphatidylinositol 3-kinase, atypical protein kinase C and protein kinase B/Akt in human diabetic muscle.Diabetologia. 2009 Jan;52(1):60-4. doi: 10.1007/s00125-008-1180-z. Epub 2008 Oct 30. Diabetologia. 2009. PMID: 18972094 Free PMC article.
-
Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): actions and defects in obesity and type II diabetes.Exp Biol Med (Maywood). 2005 Oct;230(9):593-605. doi: 10.1177/153537020523000901. Exp Biol Med (Maywood). 2005. PMID: 16179727 Review.
-
Invited review: effect of acute exercise on insulin signaling and action in humans.J Appl Physiol (1985). 2002 Jul;93(1):384-92. doi: 10.1152/japplphysiol.00043.2002. J Appl Physiol (1985). 2002. PMID: 12070228 Review.
Cited by
-
Short-term exercise training improves insulin sensitivity but does not inhibit inflammatory pathways in immune cells from insulin-resistant subjects.J Diabetes Res. 2013;2013:107805. doi: 10.1155/2013/107805. Epub 2013 Mar 13. J Diabetes Res. 2013. PMID: 23671849 Free PMC article.
-
Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle.Mol Metab. 2018 Oct;16:24-34. doi: 10.1016/j.molmet.2018.07.001. Epub 2018 Jul 25. Mol Metab. 2018. PMID: 30093357 Free PMC article.
-
Effect of lifelong football training on the expression of muscle molecular markers involved in healthy longevity.Eur J Appl Physiol. 2017 Apr;117(4):721-730. doi: 10.1007/s00421-017-3562-8. Epub 2017 Mar 1. Eur J Appl Physiol. 2017. PMID: 28251397
-
Exercise and Glycemic Control: Focus on Redox Homeostasis and Redox-Sensitive Protein Signaling.Front Endocrinol (Lausanne). 2017 May 5;8:87. doi: 10.3389/fendo.2017.00087. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28529499 Free PMC article. Review.
-
Exercise training reduces the insulin-sensitizing effect of a single bout of exercise in human skeletal muscle.J Physiol. 2019 Jan;597(1):89-103. doi: 10.1113/JP276735. Epub 2018 Nov 22. J Physiol. 2019. PMID: 30325018 Free PMC article. Clinical Trial.
References
-
- Aronson D, Boppart MD, Dufresne SD, Fielding RA, Goodyear LJ. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem Biophys Res Commun. 1998;251:106–110. - PubMed
-
- Balon TW, Zorzano A, Treadway JL, Goodman MN, Ruderman NB. Effect of insulin on protein synthesis and degradation in skeletal muscle after exercise. Am J Physiol Endocrinol Metab. 1990;258:E92–E97. - PubMed
-
- Bandyopadhyay G, Kanoh Y, Sajan MP, Standaert ML, Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-λ on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. - PubMed
-
- Bandyopadhyay G, Standaert ML, Sajan MP, Karnitz LM, Cong L, Quon MJ, Farese RV. Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase C-ζ. Mol Endocrinol. 1999;13:1766–1772. - PubMed
-
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

