Inhibiting TGF-beta signaling restores immune surveillance in the SMA-560 glioma model
- PMID: 17522330
- PMCID: PMC1907409
- DOI: 10.1215/15228517-2007-010
Inhibiting TGF-beta signaling restores immune surveillance in the SMA-560 glioma model
Erratum in
- Neuro Oncol. 2007 Oct;9(4):465
Abstract
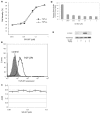
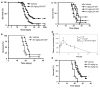
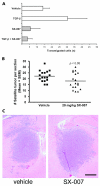
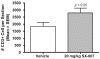
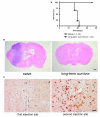
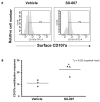
Transforming growth factor-beta (TGF-beta) is a proinvasive and immunosuppressive cytokine that plays a major role in the malignant phenotype of gliomas. One novel strategy of disabling TGF-beta activity in gliomas is to disrupt the signaling cascade at the level of the TGF-beta receptor I (TGF-betaRI) kinase, thus abrogating TGF-beta-mediated invasiveness and immune suppression. SX-007, an orally active, small-molecule TGF-betaRI kinase inhibitor, was evaluated for its therapeutic potential in cell culture and in an in vivo glioma model. The syngeneic, orthotopic glioma model SMA-560 was used to evaluate the efficacy of SX-007. Cells were implanted into the striatum of VM/Dk mice. Dosing began three days after implantation and continued until the end of the study. Efficacy was established by assessing survival benefit. SX-007 dosed at 20 mg/kg p.o. once daily (q.d.) modulated TGF-beta signaling in the tumor and improved the median survival. Strikingly, approximately 25% of the treated animals were disease-free at the end of the study. Increasing the dose to 40 mg/kg q.d. or 20 mg/kg twice daily did not further improve efficacy. The data suggest that SX-007 can exert a therapeutic effect by reducing TGF-beta-mediated invasion and reversing immune suppression. SX-007 modulates the TGF-beta signaling pathway and is associated with improved survival in this glioma model. Survival benefit is due to reduced tumor invasion and reversal of TGF-beta-mediated immune suppression, allowing for rejection of the tumor. Together, these results suggest that treatment with a TGF-betaRI inhibitor may be useful in the treatment of glioblastoma.
Figures
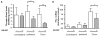
Similar articles
-
SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo.Cancer Res. 2004 Nov 1;64(21):7954-61. doi: 10.1158/0008-5472.CAN-04-1013. Cancer Res. 2004. PMID: 15520202
-
Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment.Anticancer Res. 2007 Nov-Dec;27(6B):4149-57. Anticancer Res. 2007. PMID: 18229422
-
Microglia-derived TGF-beta as an important regulator of glioblastoma invasion--an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor.Oncogene. 2008 Feb 7;27(7):918-30. doi: 10.1038/sj.onc.1210683. Epub 2007 Aug 6. Oncogene. 2008. PMID: 17684491
-
Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape.Microsc Res Tech. 2001 Feb 15;52(4):401-10. doi: 10.1002/1097-0029(20010215)52:4<401::AID-JEMT1025>3.0.CO;2-C. Microsc Res Tech. 2001. PMID: 11170299 Review.
-
TGF-β as a therapeutic target in high grade gliomas - promises and challenges.Biochem Pharmacol. 2013 Feb 15;85(4):478-85. doi: 10.1016/j.bcp.2012.11.005. Epub 2012 Nov 14. Biochem Pharmacol. 2013. PMID: 23159669 Review.
Cited by
-
Opportunities and challenges related to ferroptosis in glioma and neuroblastoma.Front Oncol. 2023 Mar 2;13:1065994. doi: 10.3389/fonc.2023.1065994. eCollection 2023. Front Oncol. 2023. PMID: 36937406 Free PMC article. Review.
-
Research Progress About Glioma Stem Cells in the Immune Microenvironment of Glioma.Front Pharmacol. 2021 Sep 23;12:750857. doi: 10.3389/fphar.2021.750857. eCollection 2021. Front Pharmacol. 2021. PMID: 34630121 Free PMC article. Review.
-
Clinical Neuropathology mini-review 6-2015: PD-L1: emerging biomarker in glioblastoma?Clin Neuropathol. 2015 Nov-Dec;34(6):313-21. doi: 10.5414/np300922. Clin Neuropathol. 2015. PMID: 26501438 Free PMC article. Review.
-
Immunocompetent Mouse Models in the Search for Effective Immunotherapy in Glioblastoma.Cancers (Basel). 2020 Dec 23;13(1):19. doi: 10.3390/cancers13010019. Cancers (Basel). 2020. PMID: 33374542 Free PMC article. Review.
-
The Dual Role of TGFβ in Human Cancer: From Tumor Suppression to Cancer Metastasis.ISRN Mol Biol. 2012 Dec 24;2012:381428. doi: 10.5402/2012/381428. eCollection 2012. ISRN Mol Biol. 2012. PMID: 27340590 Free PMC article. Review.
References
-
- Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Central Nervous System (World Health Organization Classification of Tumours) Lyon, France: International Agency for Research on Cancer; 2000.
-
- CBTRUS. Statistical Report: Primary Brain Tumors in the US, 1997–2001. Chicago, IL: Central Brain Tumor Registry of the United States; 2004.
-
- Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. - PubMed
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

