Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia
- PMID: 17505014
- PMCID: PMC1976363
- DOI: 10.1182/blood-2007-03-076844
Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia
Abstract
To determine whether the leukemia-associated Wilms tumor antigen (WT1) contributes to a graft-versus-leukemia (GVL) effect after allogeneic stem-cell transplantation (SCT) for acute lymphoblastic leukemia (ALL), we studied CD8(+) T-cell responses to WT1 in 10 human lymphocyte antigen (HLA)-A*0201-positive ALL patients during the early phase of immune recovery after SCT (days 30-120). Seven of 10 patients had detectable WT1 expression in their peripheral blood (PB) before SCT by quantitative reverse-transcription polymerase chain reaction. Using WT1/HLA-A*0201 tetramers and intracellular interferon-gamma (IFN-gamma) staining, WT1(+) CD8(+) T-cell responses after SCT were found only in patients with detectable WT1 expression before SCT (5 of 7 vs. 0 of 3; P < .05). To monitor the kinetics of WT1(+) CD8(+) T-cell responses and disease regression after SCT, absolute WT1(+) CD8(+) T-cell numbers and WT1 expression were studied for each time point. The emergence of WT1(+) CD8(+) T cells was associated with a decrease in WT1 expression, suggesting a WT1-driven GVL effect. Loss of WT1(+) CD8(+) T-cell responses was associated with reappearance of WT1 transcripts, consistent with a molecular relapse (P < .001). WT1(+) CD8(+) T cells had a predominantly effector-memory phenotype (CD45RO(+) CD27(-)CD57(+)) and produced IFN-gamma. Our results support the immunogenicity of WT1 after SCT for ALL and highlight the potential for WT1 vaccines to boost GVL after SCT for ALL.
Figures

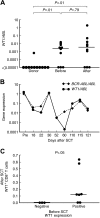
 ) and WT1-specific IFN-γ producing CD8+ T cells (
) and WT1-specific IFN-γ producing CD8+ T cells ( ).
).



Similar articles
-
Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation.Blood. 2003 Oct 15;102(8):2892-900. doi: 10.1182/blood-2003-01-0150. Epub 2003 Jun 26. Blood. 2003. PMID: 12829610
-
Graft-versus-leukemia effect with a WT1-specific T-cell response induced by azacitidine and donor lymphocyte infusions after allogeneic hematopoietic stem cell transplantation.Cytotherapy. 2017 Apr;19(4):514-520. doi: 10.1016/j.jcyt.2016.12.007. Epub 2017 Jan 27. Cytotherapy. 2017. PMID: 28139337
-
Graft-versus-leukemia effects of Wilms' tumor 1 protein-specific cytotoxic T lymphocytes in patients with chronic myeloid leukemia after allogeneic hematopoietic stem cell transplantation.Chin Med J (Engl). 2010 Apr 5;123(7):912-6. Chin Med J (Engl). 2010. PMID: 20497687 Clinical Trial.
-
Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review.
-
WT1 gene transcript assay for relapse in acute leukemia after transplantation.Leuk Lymphoma. 2004 Sep;45(9):1747-53. doi: 10.1080/10428190410001687503. Leuk Lymphoma. 2004. PMID: 15223632 Review.
Cited by
-
Analysis of Wilms' tumor protein 1 specific TCR repertoire in AML patients uncovers higher diversity in patients in remission than in relapsed.Ann Hematol. 2024 Sep 11. doi: 10.1007/s00277-024-05919-1. Online ahead of print. Ann Hematol. 2024. PMID: 39259326
-
Haematological malignancies: at the forefront of immunotherapeutic innovation.Nat Rev Cancer. 2015 Apr;15(4):201-15. doi: 10.1038/nrc3907. Epub 2015 Mar 19. Nat Rev Cancer. 2015. PMID: 25786696 Free PMC article. Review.
-
Augmentation of anti-tumor immunity by adoptive T-cell transfer after allogeneic hematopoietic stem cell transplantation.Expert Rev Hematol. 2012 Aug;5(4):409-25. doi: 10.1586/ehm.12.28. Expert Rev Hematol. 2012. PMID: 22992235 Free PMC article. Review.
-
Analysis of the functional WT1-specific T-cell repertoire in healthy donors reveals a discrepancy between CD4(+) and CD8(+) memory formation.Immunology. 2015 Aug;145(4):558-69. doi: 10.1111/imm.12472. Epub 2015 Jun 19. Immunology. 2015. PMID: 25882672 Free PMC article.
-
NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction.Biol Blood Marrow Transplant. 2010 May;16(5):565-86. doi: 10.1016/j.bbmt.2010.02.005. Epub 2010 Feb 10. Biol Blood Marrow Transplant. 2010. PMID: 20152921 Free PMC article.
References
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. - PubMed
-
- Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. - PubMed
-
- Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood. 1995;86:2041–2050. - PubMed
-
- Collins RH, Jr, Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–516. - PubMed
-
- Slavin S, Naparstek E, Nagler A, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87:2195–2204. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

