Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly
- PMID: 17502609
- PMCID: PMC1885624
- DOI: 10.1073/pnas.0703348104
Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly
Abstract
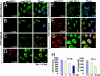
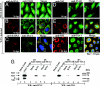
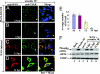
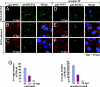
The West Nile virus minus-strand 3' terminal stem loop (SL) RNA was previously shown to bind specifically to cellular stress granule (SG) components, T cell intracellular antigen-1 (TIA-1) and the related protein TIAR. In vitro TIAR binding was 10 times more efficient than TIA-1. The 3'(-)SL functions as the promoter for genomic RNA synthesis. Colocalization of TIAR and TIA-1 with the viral replication complex components dsRNA and NS3 was observed in the perinuclear regions of West Nile virus- and dengue virus-infected cells. The kinetics of accumulation of TIAR in the perinuclear region was similar to those of genomic RNA synthesis. In contrast, relocation of TIA-1 to the perinuclear region began only after maximal levels of RNA synthesis had been achieved, except when TIAR was absent. Virus infection did not induce SGs and progressive resistance to SG induction by arsenite developed coincident with TIAR relocation. A progressive decrease in the number of processing bodies was secondarily observed in infected cells. These data suggest that the interaction of TIAR with viral components facilitates flavivirus genome RNA synthesis and inhibits SG formation, which prevents the shutoff of host translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cell proteins TIA-1 and TIAR interact with the 3' stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication.J Virol. 2002 Dec;76(23):11989-2000. doi: 10.1128/jvi.76.23.11989-12000.2002. J Virol. 2002. PMID: 12414941 Free PMC article.
-
Mutation of mapped TIA-1/TIAR binding sites in the 3' terminal stem-loop of West Nile virus minus-strand RNA in an infectious clone negatively affects genomic RNA amplification.J Virol. 2008 Nov;82(21):10657-70. doi: 10.1128/JVI.00991-08. Epub 2008 Sep 3. J Virol. 2008. PMID: 18768985 Free PMC article.
-
The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation.J Virol. 2014 Jun;88(12):6611-22. doi: 10.1128/JVI.03736-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696465 Free PMC article.
-
RNA recognition and stress granule formation by TIA proteins.Int J Mol Sci. 2014 Dec 16;15(12):23377-88. doi: 10.3390/ijms151223377. Int J Mol Sci. 2014. PMID: 25522169 Free PMC article. Review.
-
Host factors involved in West Nile virus replication.Ann N Y Acad Sci. 2001 Dec;951:207-19. doi: 10.1111/j.1749-6632.2001.tb02698.x. Ann N Y Acad Sci. 2001. PMID: 11797778 Review.
Cited by
-
P-body components LSM1, GW182, DDX3, DDX6 and XRN1 are recruited to WNV replication sites and positively regulate viral replication.Virology. 2013 Feb 5;436(1):1-7. doi: 10.1016/j.virol.2012.09.041. Epub 2012 Oct 24. Virology. 2013. PMID: 23102969 Free PMC article.
-
Dengue virus induces mitochondrial elongation through impairment of Drp1-triggered mitochondrial fission.Virology. 2017 Jan;500:149-160. doi: 10.1016/j.virol.2016.10.022. Epub 2016 Nov 4. Virology. 2017. PMID: 27816895 Free PMC article.
-
Viral modulation of stress granules.Virus Res. 2012 Nov;169(2):430-7. doi: 10.1016/j.virusres.2012.06.004. Epub 2012 Jun 15. Virus Res. 2012. PMID: 22705970 Free PMC article. Review.
-
Regulation of translation by stress granules and processing bodies.Prog Mol Biol Transl Sci. 2009;90:155-85. doi: 10.1016/S1877-1173(09)90004-7. Epub 2009 Oct 27. Prog Mol Biol Transl Sci. 2009. PMID: 20374741 Free PMC article. Review.
-
Hepatitis C virus hijacks P-body and stress granule components around lipid droplets.J Virol. 2011 Jul;85(14):6882-92. doi: 10.1128/JVI.02418-10. Epub 2011 May 4. J Virol. 2011. PMID: 21543503 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

