A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR
- PMID: 17502599
- PMCID: PMC1885573
- DOI: 10.1073/pnas.0702081104
A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR
Abstract
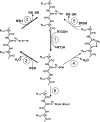
Oxidation of protein thiolates is central to numerous redox-regulated processes. Bacillus subtilis OhrR is an organic peroxide sensor that represses expression of an inducible peroxiredoxin, OhrA. Here, we present evidence that oxidation of the sole cysteine residue in OhrR leads to a sulfenic acid-containing intermediate that retains DNA-binding activity: further reaction to generate either a mixed disulfide (S-thiolation) or a protein sulfenamide (sulfenyl-amide) derivative is essential for derepression. Protein S-thiolation protects OhrR from overoxidation and provides for a facile regeneration of active OhrR by thiol-disulfide exchange reactions. The sulfenamide can also be reduced by thiol-disulfide exchange reactions, although this process is much slower than for mixed disulfides. Recovery of oxidized OhrR from B. subtilis identifies three distinct S-thiolated species, including mixed disulfides with a novel 398-Da thiol, cysteine, and CoASH. Evidence for in vivo formation of the sulfenamide derivative is also presented.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oxidant-dependent switching between reversible and sacrificial oxidation pathways for Bacillus subtilis OhrR.Mol Microbiol. 2008 May;68(4):978-86. doi: 10.1111/j.1365-2958.2008.06200.x. Epub 2008 Mar 19. Mol Microbiol. 2008. PMID: 18363800
-
Mutational analysis of active site residues essential for sensing of organic hydroperoxides by Bacillus subtilis OhrR.J Bacteriol. 2007 Oct;189(19):7069-76. doi: 10.1128/JB.00879-07. Epub 2007 Jul 27. J Bacteriol. 2007. PMID: 17660290 Free PMC article.
-
Structure of an OhrR-ohrA operator complex reveals the DNA binding mechanism of the MarR family.Mol Cell. 2005 Oct 7;20(1):131-41. doi: 10.1016/j.molcel.2005.09.013. Mol Cell. 2005. PMID: 16209951
-
PerR vs OhrR: selective peroxide sensing in Bacillus subtilis.Mol Biosyst. 2010 Feb;6(2):316-23. doi: 10.1039/b915042k. Epub 2009 Sep 18. Mol Biosyst. 2010. PMID: 20094649 Review.
-
Thiolation and nitrosation of cysteines in biological fluids and cells.Amino Acids. 2003 Dec;25(3-4):323-39. doi: 10.1007/s00726-003-0020-1. Epub 2003 Aug 21. Amino Acids. 2003. PMID: 14661094 Review.
Cited by
-
Methionine oxidation activates a transcription factor in response to oxidative stress.Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9493-8. doi: 10.1073/pnas.1300578110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690622 Free PMC article.
-
Diamide triggers mainly S Thiolations in the cytoplasmic proteomes of Bacillus subtilis and Staphylococcus aureus.J Bacteriol. 2009 Dec;191(24):7520-30. doi: 10.1128/JB.00937-09. Epub 2009 Oct 16. J Bacteriol. 2009. PMID: 19837798 Free PMC article.
-
Mfd protects against oxidative stress in Bacillus subtilis independently of its canonical function in DNA repair.BMC Microbiol. 2019 Jan 28;19(1):26. doi: 10.1186/s12866-019-1394-x. BMC Microbiol. 2019. PMID: 30691388 Free PMC article.
-
Staphylococcus aureus Uses the Bacilliredoxin (BrxAB)/Bacillithiol Disulfide Reductase (YpdA) Redox Pathway to Defend Against Oxidative Stress Under Infections.Front Microbiol. 2019 Jun 18;10:1355. doi: 10.3389/fmicb.2019.01355. eCollection 2019. Front Microbiol. 2019. PMID: 31275277 Free PMC article.
-
The Central Role of Redox-Regulated Switch Proteins in Bacteria.Front Mol Biosci. 2021 Jul 2;8:706039. doi: 10.3389/fmolb.2021.706039. eCollection 2021. Front Mol Biosci. 2021. PMID: 34277710 Free PMC article. Review.
References
-
- Imlay JA. Annu Rev Microbiol. 2003;57:395–418. - PubMed
-
- Fang FC. Nat Rev Microbiol. 2004;2:820–832. - PubMed
-
- Torres MA, Dangl JL. Curr Opin Plant Biol. 2005;8:397–403. - PubMed
-
- Mongkolsuk S, Helmann JD. Mol Microbiol. 2002;45:9–15. - PubMed
-
- Toledano MB, Delaunay A, Monceau L, Tacnet F. Trends Biochem Sci. 2004;29:351–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

